Author Details :
Volume : 5, Issue : 1, Year : 2019
Article Page : 9-15
https://doi.org/10.18231/2581-4729.2019.0003
Abstract
Introduction: Post kala-azar dermal leishmaniasis (PKDL) is a cutaneous complication of treated or subclinical visceral leishmaniasis. For confirmation, detection of Leishman-Donovan body (LDB) in slit skin smear (SSS) is needed. But it reveals a very low positivity. Since other confirmatory investigations are very costly, we studied the histopathological method as one of the accurate in the diagnosis of PKDL.
Materials and Methods: We enrolled all suspected PKDL cases (n-24) of different forms within the specified period of one year. Clinically, 5 patients had hypopigmented macular lesions, 7 patients had papules, nodules or plaque lesions and 12 patients had combination lesions. All the patients were investigated with ELISA for rK39 antibody test and SSS for LDB. We had done skin biopsy from the lesions of all cases and stained with Haematoxillin and Eosin for histopathological examination. The tissues were also stained with Geimsa and Fite Faracco stain for better accuracy.
Results: We collected 36 biopsy materials from 24 patients. Among 36 samples, 17 samples were of macular lesions and 19 samples were from papule, nodule or plaque. There were sparse lymphohistiocytic infiltrate with a few plasma cells in superficial perivascular locations in macular lesions. In papule, nodule or plaque lesions, the lymphohistiocytic and lymphoplasmacytic infiltrates were in perivascular and periappendegeal locations and extended upto mid dermis. The infiltrates were moderate to heavy according to type of elevated lesion. In some cases, the infiltrates were found in whole dermis and some formed nodular aggregate. Presence of plasma cells and hyalinization of dermal collagen were the important features in PKDL histopathology.
Conclusion: The different histopathological patterns of PKDL cases have an immense value to diagnose the case correctly.
Keywords: Post kala-azar dermal leishmaniasis, Leishman-Donovan body, Histopathology.
Leishmaniasis is a spectrum of chronic infections in humans and several animal species, caused by Leishmania, a protozoan parasite. It is transmitted by a bite of infected female sandfly of Phlebotomus genera. It was first described from Bengal, India in 1922.[1] Leishmaniasis can be categorised in three major clinical patterns, namely cutaneous, mucocutaneous and visceral. Post-kala-azar dermal leishmaniasis (PKDL) is a cutaneous complication of visceral leishmaniasis (VL) and occurs as a sequel of treated or possible subclinical visceral leishmaniasis in an endemic area. PKDL, caused by Leishmania Donovani species, occurs after few months to several years of apparent cure of VL.[2] PKDL is most commonly found in Africa particularly in Sudan and in Asia mainly in Bangladesh and to a lesser extent in India.[3] The eastern part of India, more specifically Bihar and Jharkhand states and their surrounding areas are the main habitat of PKDL patients. In India, the occurrence of PKDL is 6%-20% of VL cases.[4] The time interval to form PKDL from VL in India takes 1-20 years (mean 6.2 years).
The three major forms of PKDL are generally found clinically, namely - (1) Erythematous indurated lesions on centrofacial location mainly around the mouth and nose, (2) Multiple hypopigmented macules over trunk, extremities and face, (3) Combination of papules, nodules and plaques. Co-existence of two forms in a same patient is mostly found.[5] These clinical features are the main thing to diagnose or suspect the PKDL. The suspected cases are to be investigated by several simple tests like rK39 antibody test, direct agglutination test, slit skin smear (SSS) for Leishman-Donovan bodies (LD Bodies). The last step to diagnose the undiagnosed case is histopathology or molecular test. The molecular test is not feasible in our hospital and it is costly also. So we had done skin biopsy from lesional skin of all cases for histopathological examination (HPE). These biopsy tissues are stained with Haematoxalin and Eosin (H & E) stain and also special stain for further diagnostic accuracy. Here we studied the histopathological changes of all types of PKDL patients for a period of one year in a peripheral Medical College of West Bengal. Our aim of this study is to establish the importance of histopathology of PKDL to diagnose the case more accurately to prevent unnecessary, toxic exposure of medication.
We studied all attended PKDL cases in Dermatology out Patient Department of Malda Medical College and Hospital over a period of one year from May'17 to April'18. It is a descriptive study. The catchment area of our hospital is very wide. Apart from Malda district, the patients of surrounding districts of West Bengal, Bihar, Jharkhand and even from neighboring country, Bangladesh come here for better treatment. Due to this geographic location, we get many PKDL patients throughout the year. Within the specified duration, we examined twenty four PKDL cases for histopathological study along with other preliminary investigations. After taking written consent, all the patients were enrolled with name, age, sex, occupation, caste, history of VL, drug use or duration of VL treatment, time taken to form PKDL and duration of PKDL. [Table 1] They were sent to central laboratory of our hospital for complete blood count, liver function test, renal function test and sugar routinely. All patients were tested for rK39 antibody by rapid immunochromatographic strip assay of InBios International which is an important epidemiological diagnostic test. They were also investigated for HIV serology to rule out immunodeficiency. They were sent to Microbiology department for slit skin smear. Both Giemsa and modified Z-N stain were performed for LD bodies and Acid fast bacilli (AFB) respectively because clinically both the PKDL and Leprosy cases are very similar in presentation. This simple test can diagnose most of the leprosy cases very easily. Then we had done biopsy (3.5mm-4mm) under local anaesthesia with proper asepsis from facial lesions mainly on the day of enrolment or on next day. The biopsy materials were kept in plain vial with 10% formalin solution and sent to Pathology department for Histopathological examination. The materials were stained with H & E, Giemsa and Fite-Faraco stain after proper fixing. Thirty six biopsy materials were available from twenty four patients for evaluation. In patients of co-existed two forms, we had taken materials both forms of lesions. So out of thirty six biopsy materials, we had taken 19 materials from papule, nodule, plaque or erythematous indurated lesions and 17 materials from macular lesions.
Out of twenty four cases, eighteen cases were male and male, female ratio was 3:1. The age of patients ranged from 6-40 years with an average of 24 years. All the patients had history of Kala-Azar and sixteen patients had given adequate treatment history of VL. The lesions of PKDL started from 6 months to 31 years after VL treatment and the average was 5.1 years. The time interval from onset of PKDL lesion to attend in this hospital ranged from 6 months to 16 years with an average of 4.08 years. All patients had positive results in rapid strip test. No one was HIV reactive. Other blood reports were also normal.
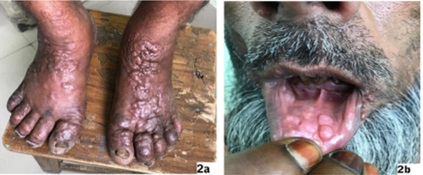
Morphological Changes: Five patients had solely hypopigmented macules [Fig. 1a], seven patients had combination of papule, nodule and plaque lesions [Fig. 1c, 1d, 2a] and twelve patients had both hypopigmented macules with papule, nodule and plaque lesions (10 cases) or with erythematous indurated facial lesions (2 cases only) [Fig. 1b]. We found extensive hypopigmented macules in two cases, extensive papule, nodule and plaque lesions in three cases and extensive mixed lesions in five cases of which macular lesion was more extensive than other form. Out of 24 PKDL cases, four patients had also mucosal lesions of which three had papulonodular lesions on inner aspect of lower lip [Fig. 2b], tongue and one patient had macular lesion on the glans penis.
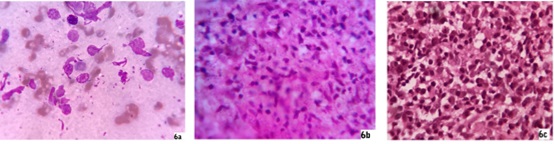
Tissue smear - Slit skin smear from lesions of all cases were done for LD bodies and AFB. Only one papulonodular case, one papulonodular lesion of mixed form and one extensive macular case showed positive LD bodies in Giemsa stain [Fig. 6a]. All smears for AFB on modified Z-N stain were negative.
Histopathological Changes: All H & E stained slides were examined thoroughly under light microscope with different magnifications including oil immersion lens.
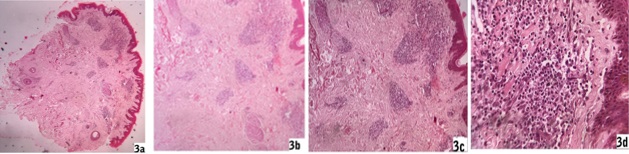
Macular lesions - Epidermis were unremarkable in most cases (12/17) though three cases had focal atrophy and two had acanthotic epidermis. Pigmentary incontinence was found in ten cases (10/17). In dermis, there were sparse perivascular lymphohistiocytic infiltrate along with a few plasma cells in the upper dermis. Periappendegeal infiltrates were also found in a few cases in mid dermis. Deep dermis and subcutaneous tissue were normal [Fig. 3a, 3b, 3c and 3d]. There was no specific difference of infiltrates between a few and extensive macular lesion cases, even between solely macular case and macular lesion of co-existed form. Giemsa stains for LD bodies were done but were unable to detect any LD body. No AFB was found in Fite stain.
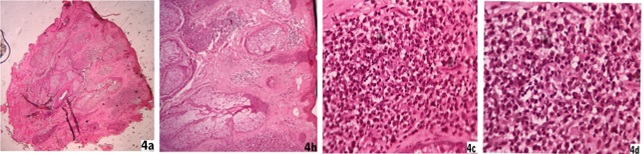
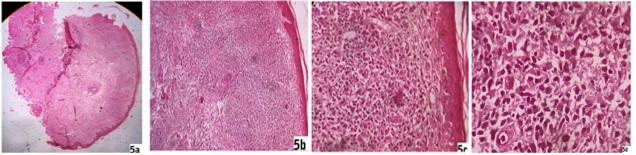
Papule, nodule, plaque lesions and erythematous indurated facial lesions - Epidermis were flattened in five patients (5/19) of nodular lesions whereas hypertrophied epidermis consisting of orthokeratosis and acanthosis were found in another eight biopsy samples (8/19) of papule, plaque or indurated lesions. Rest of six patients had both atrophy and hypertrophy of epidermis simultaneously according to sites of heavy and moderate infiltrates respectively (6/19). There were follicular plugging in most of the facial biopsy materials. Grenz zone was found in ten patients (10/19) and that included in all nodular lesions. There were profuse lymphohistiocytic and lymphoplasmacytic infiltrates extending diffusely throughout the dermis with more predilection at periappendegeal and perivascular location [Fig. 4a, 4b, 4c,4d]. We found plenty of plasma cells along with heavy lymphohistiocytic infiltrates in five cases (5/19) [Fig. 6c] and the infiltrates formed nodular aggregates in upper and mid dermis to stretch the epidermis [Fig. 5a, 5b, 5c, 5d]. Histiocytes with minimal morphological variation were found in most of the cases. But we noticed epithelioid histiocytes in three cases and foamy changes in two cases. Actually we found LD bodies in these cases. In lower dermis the infiltrates were sparse, though in two cases the heavy infiltrates were found up to deep dermis. There was no infiltration in subcutaneous tissue. There was hyalinization of dermal collagen at infiltrated areas mainly. There was no well-formed epithelioid granuloma. We did not find any specific difference of infiltrates between the mixed case and solely papule, nodule or plaque lesion. We found LD bodies within the histiocytes in five cases only (5/19) in 100 X magnification and those included the two smear positive cases [Fig. 6b]. Fite stain of all cases were negative.
 |
Click here to view |
Fig. 1: 1a, 1b, 1c, 1d
 |
Click here to view |
Fig. 2: 2a, 2b
 |
Click here to view |
Fig. 3: 3a, 3b, 3c, 3d
 |
Click here to view |
Fig. 4: 4a, 4b, 4c, 4d
 |
Click here to view |
Fig. 5: 5a, 5b, 5c, 5d
 |
Click here to view |
Fig. 6: 6a, 6b, 6c
Discussion
PKDL mostly follows after adequate or inadequate treatment of VL and it is an intermediate disease state before full recovery from VL. Classical PKDL patients are not ill, have recovered from malnutrition and usually do not have hepatosplenomegaly like VL. The skin rashes appear around the persisting dermal parasites in absence of systemic parasitaemia. PKDL is mainly restricted to follow the VL caused by Leishmania Donovani species; hence it mostly occurs in Africa and Indian subcontinent. In India, man is the only reservoir of VL. So the dermal parasites (amastigote) of the VL and PKDL patients are entered into the biting sand flies and develop into infective stage (promastigote) within the midgut of these flies. So the PKDL patients have an immense epidemiological value. It has been found that the presence of 0.5% PKDL patients can potentially succeed in making VL endemic.[6]
PKDL rarely occurs during the treatment of VL and is called as para kala-azar dermal leishmaniasis. Some of the PKDL patients have no past history of VL and these patients are mostly misdiagnosed as having other skin disorders.[7] These patients are of endemic areas and they had subclinical VL. They are about 15-20% of all PKDL patients.[8]
As we know PKDL is a dermal complication of VL and all VL patients are not converted into PKDL. So there must have some risk factors which cause this complication. The possible risk factors are previous VL treatment duration, the type of drug used, young age, malnutrition, HIV infection, genetic factors and parasite strain.[9] Nowadays, PKDL is no longer considered to be a specific antileishmanial drug dependent manifestation.[10]
In VL, it has been found that there is Th2 response, no cellular immunity and high blood level of IL-6, IL-10, TGF-beta and TNF-alpha. Following treatment, Th1 immune
response occurs and levels of IL-12 and INF-gamma become high. PKDL is an intermediate immune position between Th1 and Th2 response. So in PKDL, IL-10 in the skin persists from VL due to Th2 response and systemically the Th1 response, induced after VL therapy, persists with INF gamma production.[11],[12],[13]
Before going to the main topic, we should know the correct definition of suspected, probable and confirmed PKDL cases. The patient of Kala-Azar endemic area with a past history of Kala-Azar and specific skin lesions without any anaesthesia is called a suspected PKDL patient. When the suspected patient has rK39 antibody positivity, it is called probable PKDL. A confirmed PKDL case is the probable case with detection of LD bodies in SSS or culture or having PCR positivity for LD DNA.[14]
Because of its epidemiological significance we must treat each and every cases of Indian subcontinent. As the treatment period is long and the drugs are toxic, we have to confirm the case before initiation of treatment. For confirmation we investigated the study patients step by step according to desired algorithm proposed by the working group of PKDL in New Delhi meeting in 2012.[15] First we suspected the patient as PKDL case by asking the resident of or about migration from an endemic area and/or positive past history of VL, characteristic skin lesions of PKDL without anaesthesia. Then these patients were investigated for rK39 antibody by rapid dipstick test. After that our probable cases were investigated with SSS for LD bodies and also AFB with specific stains. Though we found very little percentage of LD body positive patients in SSS, but this SSS test has an immense value to diagnose leprosy particularly lepromatous pole by AFB positivity in modified Z-N stain. Upto this step, we found only 12.5% confirmed cases by SSS positivity only. Other tests like culture and PCR are not possible in peripheral institution. So we stepped to the next possible investigation. In our hospital, the sophisticated molecular test is not available till date. So we had done the histopathological examination of all cases to clinch the confirm diagnosis as close as possible. The probable cases were biopsied from lesional skin and thoroughly examined the histopathological features. The biopsy tissues were also stained with Giemsa and Fite stain to evaluate LD bodies and AFB respectively.
As the histopathology was the main focus of our study, we had to differentiate our cases from other similar histopathology and clinical features. There are many differential diagnosis of PKDL on clinical and histopathological points of view. Clinically the macular form must be differentiated from early stage of vitiligo, pityriasis versicolor, pityriasis alba, macular form of Hansen’s infection, chronic arsenicosis, tinea infection, discoid lupus erythematosus (DLE). Amongst these, most important differential diagnosis is the macular form of Hansen’s disease. To differentiate these two, history and clinical examination play an important role to suspect the particular disease. Slit skin smear (SSS) with stains of Giemsa and modified Z-N stain for LD bodies and AFB respectively clinch the diagnosis in most of the cases before going to HPE. In HPE of these two, the infiltrates of PKDL are mostly found around the vessels of superficial vascular plexus whereas in leprosy, the infiltrates are centered mostly around the neurovascular plexus in lower dermis.[16]Presence of plasma cells is also an important diagnostic parameter in favour of PKDL. Exhaustive search for offending organism in H and E stain and also in special stains of biopsy material are needed for further diagnosis before jump into any sophisticated investigation. In leprosy with lepromatous pole, we find AFB easily in SSS and HPE because of high bacteriological index. In macular stage of PKDL, it is difficult to find the LD bodies in both SSS and HPE in H&E stain and also in special stain. El Hassan reported that when the LD bodies are a few in number, they are mostly found just beneath the epidermis.[17] Out of 17 samples for SSS from macular lesions, we found only one positive patient (5.88%) but none had any visible LD body in HPE. Our study is slightly differ from other studies where there was no LD body in SSS.[18],[19]No LD body was found in HPE of Rathi SK et al study even in special stain but Ramesh V et al study showed LD bodies in small number of patients. Beena KR et al study could not found any LD body in HPE of macular PKDL but could able to demonstrate them after immunohistochemistry.[20] Clinically the early stage of hypopigmented vitiligo is also difficult to different from PKDL. HPE of early vitiligo shows a superficial perivascular and occasional lichenoid mononuclear cell infiltrate at the border which is very close to the macular PKDL. In that case, other investigations like rK39 antibody, LD bodies demonstration may be helpful along with good clinical acumen. Sometimes wait and watch may establish the difference. KOH smear can easily diagnose the pityriasis versicolor and tinea infection. Pityriasis alba is mostly found in children over face and upper trunk and resolves spontaneously in most cases. HPE shows orthokeratosis, focal parakeratosis, spongiosis, dilated superficial vessels with perivascular lymphohistiocytic infiltrates. These features are easily differentiated from PKDL. Classical DLE lesions are plaques with characteristic histopathology of follicular plugging, vacuolar degeneration of basal cell layer, lymphohistiocytic infiltrates and scarring.
Papule, nodule, plaque or erythematous indurated lesions of PKDL are to be differentiated from various forms of cutaneous Leishmaniasis, nodular or plaque lesions of Hansen’s infection, lupus vulgaris, DLE, secondary syphilis, neurofibromatosis etc. Cutaneous leishmaniasis is diagnosed clinically as papular lesions turn into nodules or plaques and ultimately form ulcers or verrucous growth. Histopathologically, there are areas of ulceration, pseudoepitheliomatous hyperplasia with lymphohistiocytic cells, plasma cells and neutrophilic infiltrates. In acute localized lesion, the LD bodies are plenty within histiocytes. In chronic lesion, there are epithelioid cell granulomas of tuberculoid in nature within the superficial and deep dermis.[21] There are giant cells in longstanding cases. LD bodies are found within dermal macrophages. History, clinical features and HPE can collectively differentiate it from PKDL. Histopathology of secondary syphilis consists of normal or psoriasiform epidermis with perivascular, nodular or diffuse infiltrates of plasma cells, lymphocytes and histiocytes. Clinically and serologically, it is easily differentiated from PKDL. Sometimes it is very difficult to differentiate nodular or plaque form of PKDL from nodule or plaque form of leprosy clinically. Again history, clinical examination, rK39 antibody test and SSS with proper stains will show the road map. Many cases are diagnosed by these ways. Rests are clearly diagnosed by HPE with H and E stain and also special stains. The HPE of nodular or plaque lesions of leprosy depends on the pole of spectrum. In tuberculoid pole of the disease, there are typical epithelioid cell granulomas along the neurovascular bundles with lymphocytes and Langhans giant cells. The granulomas may also found around the periappendegeal structures. In lepromatous pole, the cells of granuloma are mostly macrophages with a few lymphocytes and occasional epithelioid cells. In extreme lepromatous pole, the macrophage granulomas consist of sheets of histiocytes covering almost the entire dermis. Some cases the histiocytes are foamy in nature. These histopathological features clearly diagnose the leprosy cases. The inflammatory infiltrates have a fairly sharp margin in PKDL patients whereas the peripheral limits of infiltrates are infiltrating in nature in lepromatous leprosy.[22] If the nerve twigs are found in the biopsy field, it is invariably affected by infiltrates in leprosy. In rare situations the perineural infiltrates are found both in leprosy and PKDL, and arise diagnostic dilemma.[23],[24]So by exclusion and by the presence of plasma cells with lymphohistiocytic infiltrates, we can diagnose the PKDL cases. In our study, we were also able to demonstrate LD bodies in five patients (26.3%) out of 19 patients of elevated lesions in H&E and also Giemsa stain of HPE. Rathi SK et al demonstrated LD bodies in 40% cases in H&E stain of HPE. Other studies revealed variable degree of LD bodies positivity in H&E stain of biopsy ranging from 67-100% in nodular lesions, 36-69% in papular lesions and 7-33% in macular lesions.[25] ,[26]These huge differences in various studies prove that there is great confusion of LD bodies with nuclear debris in phagocytes. Even Giemsa stain cannot able to differentiate these two. It is difficult to recognize LD bodies confidently when the numbers are small in light microscope.[27] So sophisticated investigations like quantitative PCR, species specific PCR, antigen detection test, molecular tools, tests of cure and infectivity are needed for confirm diagnosis of PKDL and its treatment. [15] But these tests are not available in our institution. These are also very costly, not suitable for field workers. So the low cost, less invasive investigations like rK39 rapid diagnostic test, SSS, HPE with good interpretation have an immense value in this poor man’s disease. Initial screening by serology (rK39 kit test) followed by confirmatory diagnosis of PKDL with SSS examination is simple and minimally invasive procedure for epidemiological studies.[28] But different studies on SSS showed different results. Das ML et al study on SSS showed that LD bodies were found in 70% nodular lesions, 20% each in macular and maculopapular lesions.[29] Contrarily, Mathur P et al study concluded 100% positivity by SSS microscopy whereas Nandan P et al study showed only 26.67% positivity.[30],[28] Due to this huge discrepancy, we want to stick on HPE mostly, along with demonstration of LD bodies in SSS and special stain on HPE to confirm the diagnosis because the molecular tests are far away. In HPE, apart from characteristic lymphohistiocytic infiltrates of variable amount, the presence of plasma cells, hyalinization of dermal collagen and vessels wall, Grenz zone, follicular plugging clinches the diagnosis of PKDL easily in most cases. Due to unavailability of molecular test and variation of results of LD bodies detection, there is an immense importance of histopathology to diagnose correctly till date especially in peripheral teaching institution.
Conclusion
We found many PKDL cases throughout the year. We could not reach the proper diagnosis with cent percent accuracy by ongoing rK39 antibody test or LD body detection test. Other sophisticated investigations are very costly and are not available everywhere. So the histopathological examination of PKDL is very much helpful to diagnose the case correctly.
Conflicting of Interest: None.
How to cite : Kar C, Saha P, Chakrabarti N, Sarkar P, Histopathological study of post kala-azar dermal leishmaniasis and its importance for diagnosis in a peripheral tertiary institution. IP Indian J Clin Exp Dermatol 2019;5(1):9-15
This is an Open Access (OA) journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Viewed: 3536
PDF Downloaded: 681