Introduction
The field of dermatology has been revolutionised by the introduction of topical corticosteroid molecules of varying potencies owing to their quick response and effective treatment. Increasing injudicious prescriptions, use of steroids as fairness creams and indis criminate over the counter sale of steroids , are the leading causes for steroid dependence. These are the most unresolved issues that need to be controlled.
Uncontrolled, unauthorized and unsupervised use of topical preparations for wrong indications have a serious impact on the quality of life of patients especially when it is the facial skin which is involved.1 The lack of information regarding serious cutaneous effects has contributed to the count less users. A careful assessment and proper counseling of these patients and general physicians and paramedical professionals against the use of these preparations over the face is the need of the hour.
Materials and Methods
This was a prospective questionnaire type of study conducted in the Dermatology Out Patient Department of Adichunchunagiri Institute of Medical Sciences in a duration of 1 year, from 2018 to 2019. Data was collected from patients who had used topical steroids incorrectly and inappropriately over face.
Inclusion criteria
All patients complaining of facial dermatoses (excluding nevi, seborrhoeic keratosis, adnexal tumors, dermatosis papulosa nigra ) reporting to the investigator were asked regarding application of topical steroids over face. The patients who applied topical steroids for various conditions and overused TC prescribed by a physician beyond the time limit of 1 month of continuous application or more than 3 months of intermittent application, or who applied steroid on the face and presented with one or more side effects of these agents as the chief complaint were included.2 Patients fulfilling the study criteria were registered fo r further workup as per the proforma.
Exclusion criteria
Patients who had cutaneous adverse effects suggestive of topical corticosteroid abuse without details of agents were excluded. Those who were not consenting to answering the questionnaire or patients with comorbidities that resembled or that could cause changes similar to topical steroids side effects (polycystic ovaries/Cushing's syndrome/thyroid disorders) were excluded from the study.
Procedure of study
A detailed history was taken based on the pretested and structured questionnaire. The investigator documented the potency of the corticost eroid used, by seeing the prescription or used tube or by showing samples of popularly used preparations. The type, potency, frequency of application, duration of therapy, indication, and source of information for its use were recorded. Photographic documentation of the patients were done. Detailed dermatological examination of each patient was done with emphasis on the morphology, pattern and distribution of skin lesions.
Results
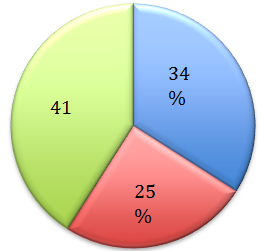
A total of 100 patients using topical corticosteroids were screened at the Dermatology Out patient of Adichunchunagiri Institute of Medical Sciences. Out of 100 patients, preponderance were seen in females (M :F = 1:2.5). Of the 100 patients in the study group, the largest number was in the 21-30 years age (35%). Most common group which abused were students (25%), followed by housewives (22%), farmers (22%), laborers (12%) and others (17%). Majority of the patients (76%) used mid potent steroids, while 24% of them used, potent steroids on their face. Details of the most commonly used brands and their composition are shown in (Table 1). Bet amethasone valerate 0.1% cream was the most commonly misused topical steroid. However steroid with antibiotic and antifungal combination was misused most commonly (41%), followed by steroid alone (34%) and triple combination (25%) of hydroquinone, tretinoin and steroids (Figure 1).
Table 1
Brand names and composition of topical corticosteroid containing products used on face in our current study
Figure 1
Pie diagram showing relative abuse of topical corticosteroids with and without combination.
Most of them had bought topical corticosteroids without prescription (75%) (Figure 2) The topical preparation was recommended by friends (46%), general practitioners (21%), neighbours (18%), family members (6%), beauticians (2%). Few took self medications (4%) and few misused the prescriptions given by dermatologists(4%) (Table 2).
Most common indication for application was acne (35%), followed by depigmentation (31%) and melasma (28%) (Table 3)
Table 2
| Source of medication | Number of patients/ Percentage |
| Dermatologist | 4 |
| General practitioners | 21 |
| Family | 6 |
| Friends | 46 |
| Neighbour | 18 |
| Beauticians | 2 |
| Self | 2 |
Source of medication in patients using topical corticosteroids on face
Table 3
| Indication for TC misuse | Number / % |
| Acne | 35 |
| Depigmentation | 31 |
| Melasma | 28 |
| Fairness | 5 |
| Hypopigmentation | 1 |
Indication for topical steroid abuse
About 4% patients used the preparation for <1 week, 19% for less than 1 month, 22% of them used it for duration of 1 month to 3 months, 17% for a duration of 3 months to 6 months, 9% for 6 months to 1 year and 29% patients for more than a year.
(Table 5)
Table 4
| Duration of use | Number (%) |
| <1 week | 4 |
| 1 week to 1 month | 19 |
| 1-3 months | 22 |
| 3-6 months | 17 |
| 6 months to 1 year | 9 |
| >1 year | 29 |
Duration of topical steroid abuse in our study
The following were the indications for withdrawal among 64% of the patients, the most common being acne in 26% patients, pigmentation in 20%, erythema in 12%, pruritus in 3%, dryness in 2% and burning sensation in 1% of patients. Presenting cutaneous features to the hospital noted were acne, pigmentation, erythema, hypertricosis, scaling, telangiectasia, perioral dermatitis, atrophy, photosensitivity and dryness. 58% of patients had combination of above symptoms. Among the other 42%, acneiform eruption was the most common presentation in 19 patients (45.2 %), pigmentation in 16 patients (38.1%), erythema in 5 patients (11.9%), hypertrichosis in 1 patient(2.4%) and atrophy in 1 patient (2.4%).
Discussion
Topical steroids in dermatology were first used in 1952 by Sulzberger and Witten. They published an article on the effect of topically applied compound F (hydrocortisone) in selected dermatoses.3 There after, many other topical steroids were introduced with varying potencies and formulations. These drugs have greatly helped dermatologists and revolutionized the treatment of various cutaneous disorders. Them isuse and abuse of these drugs by both non-dermatologists and patients were contributed to the dramatic symptomatic relief from these drugs. The free availability of these drugs as fairness or cosmetic creams, which are available as an over-the-counter medication was the main reason for the misuse in our country.4
In our study, a characteristic female preponderance was seen (M: F=0.4:1). Our observation was in concordance with the studies conducted by Manchanda K et al.(M:F=0.4:1) and Chohan SN et al.(M: F ratio=0.3:1).4,5 The obsession of Indian population towards fair complexion and the self conscious nature of females could have been the reason for female outnumbering males in abuse of topical corticosteroids. In contrast to our observation, a study by Meena S et al. showed a male preponderance with a M: F ratio of 1.7:1.6
In our study, the observation of TC abuse was more common in students (25%). Similar results were obtained from a study by Chohan SN et.al, where students (41%) misused topical steroids the most.4
The age of patients in our study ranged between 7 years and 59 years. Most common age group affected was 21–30 years (35%). This was in concordance with the study conducted by Bain P et.al (21-30 years - 49%) and by Chauhan A et al (20-39 years- 66.7%).7 This was contrary to the observation by Manchanda K et.al (11 to 20 years- 55%).5
Based on American classification of potency of topical steroids, in the present study we found that, most TC abused were mid potent TC, which was in concordance with the observation by Rathod PR et al.8,9 In contrast, in a study by Meena S et al, potent corticosteroid (clobetasone propionate 0.05%) was most commonly abused.6
In our study, Betamethasone valerate 0.1% cream was the most commonly misused topical steroid and Betnovate was the most common brand name. However steroid with antibiotic and antifungal combination was misused most commonly by 41%. 34% of patients used steroid alone and 25% used triple combination of hydroquinone, tretinoin and steroids.
The sources of pre scription for our patients were from a non-physician source. Of these, in 46% cases were recommended by a friend, 25 % by doctor (21% by general practitioners, 4% by dermatologists), 18% by neighbours, 6% by family members, 2% by beauticians and 4% by self from pharmacy. Most of them bought medications without prescription (75%). Our obsevations were in concordance with study by Dey VK et al and Rathod PR et al.8,10 In few other studies, this incidence of suggestion by friends was quite high at 64% in a study from Pakistan Chohan SN et.al and at 51% by Brar KB et al4,11 This calls for a strict regulation of sale with prescription accepted only from qualified persons. Furthermore, non-dermatologist need to be educated about adverse effects of TCs as they are unaware of havoc caused by misuse of steroids used topically.
In our study the most common indication for TC use was acne (35%). Studies done by Chohan SN et al (51.5%) and Ambika H et al12 (41%) have reported acne as the most common reason for use of TC.4,11 In contrary, Dey VK et.al (50.39%) and Sarswat et al (29%) have reported melasma as the most common reason for TC use.10,13
In the current study, majority of the patients (29%) misused TC for more than a year while 4% patients used the preparation for <1 week, 19% for less than 1 month, 22% of them used it for duration of 1 month to 3 months, 17% for a duration of 3 months to 6 months, 9% for 6 months to 1 year. Contrary to our study, a study conducted by Saraswat A et al, showed that majority of patients (117;27%) used the topical steroids for a period of 1 month to 3 months, with only 92 (21%) patiets using TC for more than a year.13 In a study by Bains P et.al duration of application of topical corticosteroids was <6 months (45%) in majority of patients.14
Anti-inflammatory effect contributes to majority of action of topical corticosteroids. They also possess potent anti-pruritic, immunosuppressive, melanopenic and atrophogenic effects on the skin. With misuse of topical steroids, all these can lead to significant local adverse effects. Use of topical steroids on the face produces peculiar adverse effects like steroid rosacea, acneiform eruption, hypertrichosis, atrophy, telangiectasia, diffuse facial erythema, pruritus, burning sensation, irritation, photosensitivity, demodicidosis, hyper/hypopigmentation, irritant contact dermatitis, perioral dermatitis, tachyphylaxis, stellate pseudoscars, purpura and milia.4,5,13,14 Off late a new condition termed as topical steroid-dependent face has been documented (described as steroid addiction, red faces yndrome by different authors) characterised by severe rebound erythema, burning and scaling on the face, persistent pin point erythematous papules, pustules and nodules along with telangectactic vessels on a firm edematous skin on any attempted cessation of application after prolonged topical steroids use on the face.14,15 On withdrawal of topical steroids, there occurs a sudden ces sation of vasoconstrictor effect and thereby release of proinflammatory cytokines and this causes the rebound phenomenon and topical steroid dependant facies.14
Patients presented to the hospital with the cutaneous complaints of acne, pigmentation, erythema, hypertrichosis, scaling, telangiectasia, perioral dermatitis, atrophy, photosensitivity and dryness. We observed in the present study that 64% of patients had withdrawn topical corticosteroids and the most common indication for withdrawal was acne (26%), pigmentation in 20%, erythema in 12%, pruritus in 3%, dryness in 2% and burning sensation in 1% of patients. 58% of patients had combination of above prsenting cutaneous features. Among the other 42%, acneiform eruption was the most common presentation in 19 patients(45.2%). Similar results were obtained in studies conducted by Dey VK et.al (144; 38%), Saraswat et.al (249; 57.5%).10,13 Different from our study, the most common side effect encountered by Chohan SN et.al in their study was facial erythema (51.8%).2
Figure 3-5: Adverse cutaneous effects of topical corticosteroids on face
Figure 3
Diffuse erythema and telangiectasia in a female who was on topical corticosteroid abuse for more than a year

There is little awareness about the adverse effects of these drugs among the general public. Moreover, they are sold as an over the counter preparation without medical prescription or control.
Table 5
Parameters assessed in our study in comparison with various other previous studies
The factors contributing to this misuse were pharmacists, paramedical personnels, the patient hims elf and family. General physicians and even few dermatologists play a role in this misuse, emphasiz ing the fact that they no not specify the adverse effects and proper dosing of topical corticosteroids to the patients. The prescriptions also get misused to get repeated refills from the pharmacist.
Conclusion
Our study proves that topical steroid abuse is very common in our population. Use of topical corticosteroids as an unwarranted cosmetic cream is quite common in India. It needs multi -sectorial interventions, involving legal, educational, and managerial approaches to overcome it. The urge to use these products by the population who are unaware of the adverse effects is regrettable. This situation is likely to get worse until control measures are taken on multi-dimensional fronts. There is a need to regulate the managerial sector with appropriate supervision and oversight and a need to change the public obsession towards fair skin tone and to accept their natural ski n tone despite the social non-compliance to such ideas. Equally important is the role of primary health care providers. There is a need to educate the primary health care workers about the adverse effects of topical steroid abuse, especially on the face and encourage them to seek a dermatology consultation for suitable and safe alternatives.1,9
As indicated by the data in our study, the problem of topical steroid misuse is already significant and needs immediate action to be taken on all possible fronts. If not, there will be worsening of this scenario and we may soon be facing a disastrous inflow of these unfortunate patients to our clinics and hospitals.1



