Introduction
Dermatophytosis caused by Trichophyton, Microsporum, and Epidermophyton is the most common fungal infection affecting 20%–25% population globally, with varying geographic distribution.1, 2 Due to India’s hot and humid climate, there has been a rampant increase in the cases of dermatophytosis and atypical presentation in recent years. 3, 4 In addition, the recommended treatment of commonly prescribed antifungal agents no longer seems to be valid in the current scenario, resulting in treatment failures and relapses when given in conventional doses and for standard duration. 5 Hence, the management of dermatophytosis is becoming more subjective in order to overcome these challenges. 6, 7 Moreover, the choice of therapy is further influenced by multiple factors like simultaneous involvement of extensive body area, hair follicles and a previous history of treatment failures, recurrences and relapses. The combination therapy is a well-established concept of using synergistic and additive effects of two or more drugs to improve therapeutic efficacy and overcome drug resistance.8
Sahoo et al.9 and Murlidhar et al.10 in their comprehensive reviews recommended the use of a combination of topical and systemic antifungals in the management of patients with large lesions or recalcitrant tinea infections. Authors also commented that while using combination therapy, drugs from two different classes should be used for wider coverage, synergistic or additive action and to reduce the chance of resistance.
For the management of dermatophytosis, Terbinafine is considered to be a first‑line drug due to its favourable mycological and pharmacokinetic profile.11 It acts by inhibiting the enzyme squalene epoxidase, thereby inhibiting ergosterol synthesis.12 Till recent years, the drug was consistently effective with cure rates of >90% achieved at doses of 250 mg once a day for two weeks.11, 12 But recently, due to the overuse of the drug, there has been an increase in the incidence of terbinafine resistance resulting in increasing numbers of clinical failures and relapses.13, 14 Hence it is advisable to use higher dose of terbinafine as seen in an article by Murlidhar et al.10 In a recent study, Terbinafine was reported to be efficacious and safe in the management of dermatophytosis at higher doses of 500 mg/day.15
Itraconazole is another antifungal drug which acts by inhibiting ergosterol synthesis. It has shown good results in the treatment of dermatophytosis at doses of 100 mg once a day for two weeks and with 200 mg once a day for seven days.16, 17 But due to frequent relapses at short intervals, some physicians in India have used it in doses of 200 mg once a day for prolonged periods.7, 18 High dose of itraconazole may not be beneficial due to non-linear pharmacokinetic property.10
Ciclopirox Olamine (CPO), a hydroxypyridone derivative has been recently approved in India. It differs in structure and mechanism of action from the other known antifungal agents.19 It acts through the chelation of polyvalent metal cations, such as ferric (Fe3+) and aluminium (Al3+), thereby causing inhibition of metal-dependent enzymes (cytochromes, catalase, and peroxidase) leading to the disruption of cellular activities such as mitochondrial electron transport processes, energy production, and nutrient intake across cell membrane.20 It also alters membrane permeability causing blockage of intracellular transport of precursors.
Due to widespread resistance to various antifungal agents and a high relapse rate when used in conventional doses, there is a need to find an effective first‑line therapy for the management of dermatophytosis to achieve the maximum results with fewer relapses. Some recent trials compared the efficacy of terbinafine and itraconazole but in standard doses and duration.16, 21 Recently, a combination of itraconazole and terbinafine has also been studied22 suggesting need of either high dose or combined dose of different systemic antifungals in current settings in India. But there is no any study comparing high dose of terbinafine against standard dose of itraconazole. Hence, the present study was conducted to compare the effectiveness and safety of combination of high dose of oral terbinafine and CPO versus standard dose of itraconazole and CPO in the management of tinea corporis et cruris.
Materials and Methods
It was a prospective, observational study conducted in the dermatology outpatient of a tertiary care hospital in Ahmedabad, Gujarat. Clinically confirmed cases of tinea corporis et cruris were recruited for the study and followed up for 8 weeks, till the completion of their treatment. All consenting patients, in the age group of 18-65 years, who were diagnosed by the dermatologist as suffering from tinea corporis et cruris were included in the study, irrespective of the presence and extent of dermatophytosis in other regions of the body. Patients who were pregnant, lactating, non-consensual, as well as those who had a history of anti-mycotic treatment within 2 weeks prior to baseline visit were excluded from the study. The patients were randomly allocated either to Itraconazole 100 mg twice a day (Group I) or Terbinafine 250 mg twice a day (Group II) for four weeks. Both the groups received additional topical Ciclopirox Olamine cream for six weeks along with anti-histamines.
Mycological and clinical assessments
During the screening visit, a detailed medical history was obtained, and a thorough examination was performed. Various clinical signs and symptoms were rated according to a four-point scale from 0-3 (0=absent, 1=mild, 2=moderate and 3=severe). After commencement of the therapy, patients were followed up at a two-week interval up to six weeks (end of treatment) and at eight weeks for any residual changes. KOH examination was done at the time of enrolling the patient and at the end of the sixth week. Fungal culture was done in all KOH positive patients only at the beginning of the therapy. Liver function tests were done at the start of the therapy and at the end of the fourth week. At each visit, a clinical assessment was made. The therapeutic efficacy was evaluated at six weeks. Patients were considered cured when there was an absence of any signs and symptoms (scaling, erythema, and pruritus), and negative KOH. The study was approved by the Institutional Ethics Committee, and informed consent was taken from all patients before recruiting.
Statistical analysis
Descriptive statistics were used to summarise the effectiveness and safety endpoints using Graphpad Prism version 8 (San Diego, California: GraphPad Software Inc., 20057). Quantitative variables were analysed using means and standard deviations, while categorical variables were analysed using frequencies and percentages. For follow-up measurable data, repeated measure ANOVA was applied to see the trend of mean values over the period. At those endpoints, unpaired t test was applied and p values ≤0.05 were considered as statistically significant.
Effectiveness Assessment
Primary effectiveness endpoint
The primary effectiveness endpoint was the percentage of patients achieving complete cure at the end of the treatment period from the baseline. Complete cure was defined as the patients achieving both clinical cure and mycological cure at the end of the treatment.
Secondary effectiveness end point
Secondary effectiveness endpoints were
The percentage of patients achieving clinical cure at the end of the treatment period. Clinical cure was defined as absence of all symptoms [scaling, erythema and pruritus] at the end of the treatment.
The percentage of patients achieving mycological cure at the end of the treatment period. Mycological cure was defined as negative microscopy under potassium hydroxide (KOH) examination at the end of the therapy.
Improvement in total symptom score from the baseline in each visit
Results
A total of 80 patients were randomly assigned treatment and included in the study. Four patients were excluded from the study because of irregular follow-up. Hence, a total of 76 patients were enrolled in the final study. Thirty eight patients were treated with itraconazole and 38 with terbinafine.
hows the demographic profile of patients, the diagnosis and theculture reports in both the groups. The average age of the patients was 38.28 and 33.25 years, respectively, in Group I and Group II. There were 20 males (53%) and 18 females (47%) in Group I, while Group II had 17 males (45%) and 21 females (55%). All the patients were co-prescribed CPO and anti-histamines.
The baseline KOH examination was positive in 34/38 patients in Group I, whereas the same was positive in 33/38 patients in Group II. Fungal culture was positive in all KOH positive patients of Group I but in Group II, only 30 patients had positive fungal culture.
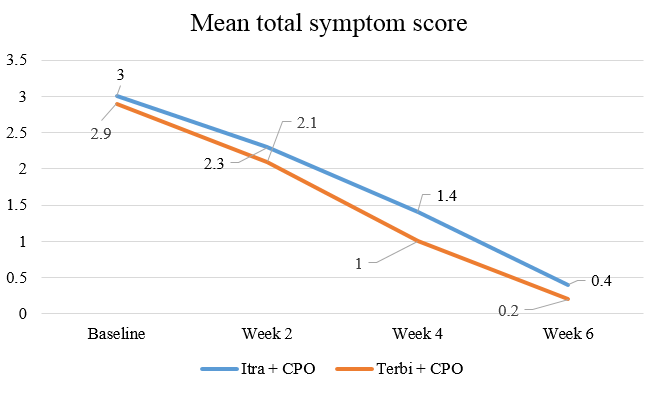
At the end of six weeks, there was a statistically significant improvement (p value<0.05) in the total symptom score (erythema, scaling, and pruritus) in Group I as well as in Group II compared to baseline [Figure 1]. The significant improvement started from 0-2 weeks and then persisted till the end of the treatment in both the groups. On comparing the groups, there was a significant improvement in the total symptom score at the end of four weeks (p value<0.05) but no statistically significant change was observed at the end of 6 weeks (p value>0.05).
Table 1
Demography, diagnosis and culture report in both the groups
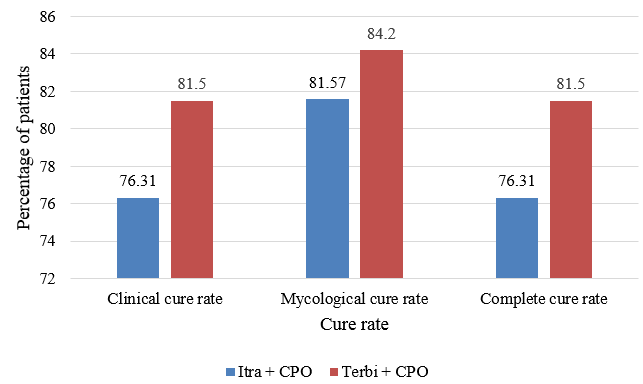
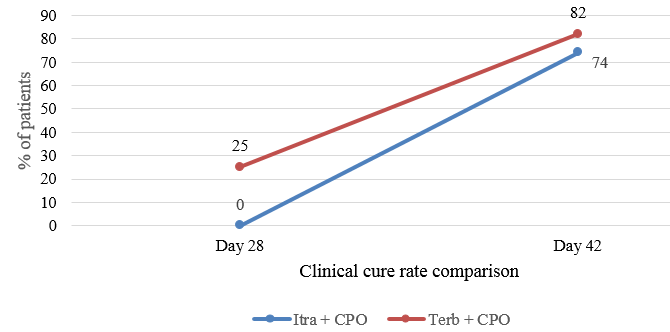
Mycological cure was achieved in 31 patients (81.57%) in Group I and 32 patients (84.2%) in Group II at the end of six weeks, whereas complete cure was achieved in 29 patients (76.31%) and 31 patients (81.5%) in Group I and II respectively at the end of six weeks. Twenty five percent of the patients achieved clinical cure at the end of four weeks in Group II which increased to 82% at the end of six weeks (Figure 2).
At the end of four weeks, the clinical cure rate was significantly better in the terbinafine group as compared to the itraconazole group. But no statistical difference was observed at the end of six weeks. Tolerability and compliance to the drug were similar in both the groups.
None of the patients showed any significant side effect in both Itraconazole and Terbinafine groups. No significant change in LFTs was also observed in both the groups during the study period.
Discussion
In recent years, the medical fraternity in India has been observing an increase in the prevalence of dermatophytosis and resistance to conventional dosage of anti-fungal drugs. This change in the clinical scenario with increasing frequency of treatment failures has given rise to the search for an effective first‑line treatment strategy that brings about rapid and complete clearance of dermatophytosis. Based on initial studies and recommendations, topical antifungals are the first line drugs in the management of dermatophytosis. However, in the current clinical scenario in India, patients with large lesions or multisite dermatophytosis, only topical therapy fails to clear the lesions, leading to treatment failures and relapses. In such patients, systemic therapy is often recommended. Recently, Sahoo et al. 9 and Murlidhar et al. 10 in their comprehensive reviews recommended the use of a combination of topical and systemic antifungals in the management of patients with large lesions or recalcitrant tinea infections. Authors commented that while using combination therapy, drugs from two different classes should be used for wider coverage, synergistic or additive action and to reduce the chance of resistance.
Ciclopirox Olamine (CPO) is a hydroxypyridone derivative that differs in structure and mechanism of action from the other known antifungal agents. 19 It acts through the chelation of polyvalent metal cations, such as ferric (Fe3+) and aluminium (Al3+), thereby causing inhibition of metal-dependent enzymes (cytochromes, catalase, and peroxidase) leading to a disruption of cellular activities such as mitochondrial electron transport processes, energy production, and nutrient intake across cell membranes. 20 It also alters membrane permeability causing a blockage of the intracellular transport of precursors. CPO is widely available in nail lacquer formulation but has been recently approved in India in cream formulation.
Systemic anti-fungal agents such as griseofulvin, terbinafine, fluconazole, and itraconazole have been known to be active against dermatophytes, terbinafine being the only fungicidal drug. 23, 24 Among these, itraconazole and terbinafine are more often prescribed compared to griseofulvin and fluconazole, probably because the latter require a longer duration of treatment. 25
In the past, Terbinafine, in the dosage of 250 mg/day, has shown consistent efficacy against dermatophytosis, achieving more than 90% cure rates at a dose of 250 mg/day when administered for two weeks. 11, 12 However, recently, an increase in the incidence of terbinafine resistance has resulted in treatment failure. 14 Although resistance to terbinafine in dermatophytosis is not common in clinical practice, it has been reported in clinical isolates by a few authors.26, 27
It has been seen that ineffective drug concentration in skin tissues may lead to anti-fungal resistance.28 Hence, a higher concentration of terbinafine 500 mg/day has seen found to be more effective in some clinical studies.15, 29, 30 Itraconazole is a triazole antifungal drug which is also increasingly being used as a first‑line drug for dermatophytosis, but it is being given for longer periods as compared to before.6, 18
Though combination therapy is widely practised in India, no much of the literature is available regarding the effectiveness of the combination therapy. Only one study is available indicating efficacy of combination therapy.31 As per that study, terbinafine with sertaconazole achieved better efficacy than itraconazole and sertaconazole combination though results are not statistically significant. Secondly, there is still the question on the right combination of systemic and topical anti-fungal agents. Hence we conducted this study to compare the effectiveness and safety of itraconazole + CPO vs. terbinafine + CPO combination to better understand the outcome of the result. As mentioned earlier, itraconazole and terbinafine are commonly used systemic anti-fungal agents. CPO was selected in view of different classes of mechanism of action and its recent introduction in India.
In our study, both the combination therapy options were efficacious in the management of dermatophytosis. Although the patients in Group II achieved clinical cure much faster (Figure 3) as compared to Group I, there is no statistical difference in the complete cure at the end of six weeks.
A recent study by Majid et al. 14 could achieve only 43% cure rate after two weeks of daily 250mg terbinafine oral treatment in dermatophytosis. This recent decrease in the clinical efficacy is well corroborated by an upsurge in the cases encountered by dermatologists in daily clinical practice along with a failure to respond to the standard oral terbinafine therapy. Another study showed the mycological cure rate of terbinafine as 74% 21 and 71%. 16 In another study, three-week therapy of terbinafine 250 mg/day showed a 35% cure rate, which was even lower as compared to other studies. 22 In all these studies, terbinafine 250 mg/day was used. But in our study, we have used terbinafine in the dose of 500 mg/day, which was well supported by some recent studies.
In a recent study, three-week treatment with itraconazole 200 mg/day showed a cure rate of 50% 22 which was lower as compared to previous studies showing variable cure rates of 80-92%. 16, 21 These variable results of clinical efficacy of itraconazole are well evidenced in some of the literature. In our study, the mycological cure rates in Group I and II were 82% and 84% respectively. Though the mycological cure rate is similar to recently done studies in the itraconazole group, in the terbinafine group, it is better than previous studies. This clearly indicates the need of using a higher dose of terbinafine along with a topical anti-fungal agent. Though many studies showed resistance or no clinical response to terbinafine, our study showed reverse results.
Similarly, the clinical cure rate in the terbinafine group was much better than the itraconazole group at week four. This could be due to the fungicidal property of terbinafine and its ability to persist in stratum corneum for several months after stopping the treatment.32
In the standard dosage, terbinafine and itraconazole have been used extensively for the treatment of dermatophytosis and have been found to be safe and well tolerated.6, 33 In the present study, the duration of the combination therapy was only four weeks but all the patients continued with topical for another two weeks. At the end of four weeks, patients in the terbinafine group showed good clinical resolution but in the itraconazole group, there was partial clinical response. But at the end of six weeks, patients in both the groups showed marked improvement indicating beneficial extended use of topical therapy. This indicates the need of monotherapy with topical for an extended duration in order to achieve higher cure rates in the treatment of dermatophytosis as suggested by Verma et al.3 In the present study, none of the patients in either group reported any adverse events.
The conventional systemic terbinafine and itraconazole monotherapy regimens at standard doses for two weeks duration have shown high treatment failure and recurrence rate in the present scenario. Though better results can be achieved by extending the dose and duration of systemic anti-fungal agents up to four weeks, a comparable cure can be achieved by combining with topical of different classes of anti-fungal agents with extended use. This combination can thus limit the duration of the treatment. In the present scenario, antifungal combination therapy appears to have a promising future in the prompt management of dermatophytosis.
Limitations of our study include its small sample size and short duration of follow-ups. Due to short duration of the follow-ups, we could not evaluate patients’ relapse rates. Additionally, this study was conducted at a single centre and hence these findings cannot be generalized. For this purpose, further multicentre studies with larger sample size are required.