Background
Vitiligo is an acquired condition with loss of pigmentation in skin of varying patterns, and with lesions that range from small macular patches with scalloping borders to near-total depigmentation of body. The disorder affects nearly 1%–2% of global population irrespective of race and ethnicity and the highest incidence recorded in Indian subcontinent followed by Mexico and Japan.1
Indian studies have reported the incidence of vitiligo to be 0.5 % -4 % in general population with Gujarat and Rajasthan states being with the highest prevalence ~ 8.8%. 2 The proportion of patients with positive family history vary across regions and range from 6% to as high as upto 30% patients while as high as 40% in an Indian study. 1 Vitiligo affects both the genders equally at any age and onset of disease peaks around 10-30 years of age. The prevalence increases with the increase in age. Disease imposes grave psychological burden as well due to cosmetically disfiguring problem.
The etio-pathogenesis of vitiligo is multifaceted with a complex interplay between genetic and non-genetic factors. 3 Several postulations regarding the melanocytes destruction, as a result of autoimmune, cytotoxic, biochemical, impaired oxidant-antioxidant, and viral, neural processes in genetically predisposed individuals have been proposed, yet the underlying mechanisms remain not completely understood. This poses a problem in selection of treatment modalities.
To explain vitiligo, among many etiological hypotheses put forward, the most compelling one is a combination of genetic and immunologic factors, which interact with each other resulting in an autoimmune melanocyte destruction. Ramaiah,2017) suggested that all the above theories are intertwined along with growth factor deprivation theory (Ramaiah et al. 1989). Until now, the autoimmune hypothesis of vitiligo etiology seems to be the most preferred. Additionally, deficiency of basic fibroblast growth factor (bFGF) have found significant role in Melanocyte homeostasis. 4
Vitiligo is a progressive disease and stabilization of disease process is the first step in management, in stable vitiligo main focus is on melanocyte stimulation to induce repigmentation of patches. Management of vitiligo is difficult and often prolonged. The selection of right treatment modality is necessary to achieve the desired repigmentation. Although several interventions are available to treat vitiligo patients, no definite cure has been developed yet.
Current topical treatment modalities for management of localized vitiligo lesions comprise of immunomodulators with moderate efficacy and remission induced is short lasting. Newer topical modalities like bFGF (a melanocyte mitogen) related decapeptide have provided promising therapeutic benefits by repigmentation of vitiligo lesions. While stabilization of disease process is the first step in management, in stable vitiligo focus is on melanocyte stimulation to induce repigmentation of patches.
Indian dermatologists, who have been prescribing bFGF related decapeptide (bFGFrP) solution along with tacrolimus ointment, suggested clinically observable efficacy and improved patient outcome. However, there were no published clinical data available to substantiate their claims about benefits of the combination of these two therapies.
Moreover, based on our current understanding of vitiligo pathogenesis, as a successful strategy to treat vitiligo we need to incorporate distinct approaches: regulating the autoimmune response, and stimulating melanocyte regeneration and migration. Existing topical tacrolimus monotherapy partially addresses these needs, however after combination with bFGFrP, the therapy may synergize to produce a better overall faster and superior response.
Hence, the current study evaluated the efficacy as well as overall improvement in response rate of the combination– on criteria like rapidity of re-pigmentation, efficacy and safety benefits of the combination of bFGFrP with Tacrolimus 0.1% ointment in patients of stable vitiligo.
Study Objectives
To compare the efficacy of bFGFrP and tacrolimus ointment in stable vitiligo patients, with tacrolimus ointment monotherapy in improving the extent of repigmentation of target lesions after 12 months of treatment.
To compare the efficacy in improvement of grades of repigmentation and patient global assessment of improvement of vitiligo among the two groups.
To evaluate safety and tolerability of the combination therapy.
Materials and Methods
This study is phase IV, open label, randomized, non-comparative, prospective, multicenteric (patients were recruited from 7 sites across India), interventional study and was conducted in compliance with Good clinical practice guidelines. Ethical clearance was obtained and informed consent was obtained from the participating patients. Adult patients with stable vitiligo, affecting less than 10% of body surface area were enrolled in the study after their written, informed consent.
Total 120 adult patients of age range 18-65 years; with clinically diagnosed stable vitiligo (without any new lesions and no progression of existing lesions ≥ 6 months) over the body surface area ≤ 10% were included in the study and randomized. The lesions 2 × 2 cm in greatest dimension served as target lesions, with no leukotrichia. The patients were randomized into two groups in 1:1 ratio, with 60 patients in each group.
The total duration of treatment was 12 months with monthly follow up visits. The test medicine preparation is Melbild (bFGFrP solution 0.1%) marketed by Alkem Laboratories Ltd. The treatment schedule consisted of bFGFrP solution 0.1% at bedtime daily on target lesion followed by morning exposure to sunlight for 5-10 minutes between 11 am-3pm daily while Tacrolimus ointment 0.1% was applied twice daily for total of 12 months duration.
Primary end point was improvement in extent of repigmentation (EOR) of target lesions while improvement in grade of repigmentation (GOR) at the end of the study and patient global assessment (PGA) were secondary end points. Efficacy assessments were done after 6 months as well as at the end of study (12months). Safety assessment with incidences of adverse events and serious adverse events was also noted throughout the study.
Study Analysis
The interim analysis was planned and completed at the end of 6 months (Shah et al. 2019).5 All patients completing the study were taken for final analysis.
Data was presented in mean ± SD for qualitative data and as number (% of total) for categorical data. Comparison of PGA scores (categories), extent of repigmentation (EOR) and grade of repigmentation (GOR) were analyzed by Friedman test and overall trend comparison was done across all visits. Chi square test was used to compare percentages of all efficacy variables between two groups. Fisher's exact probability test was applied to compare percentages between two groups for two by two data. Level of significance was taken as p ≤ 0.05 [S = significant, NS = not significant, DF = degrees of freedom]
Out of 120 randomized patients, 54 from the combination group (bFGFrP + T), 51 from tacrolimus monotherapy group completed the study, and data was analyzed. [Table 1]
Results
The demographic variables were comparable in both groups at baseline.
As published in our interim analysis, at the end of the 6 months of treatment period and regular follow up, significant improvement was seen in EOR and GOR as well as in PGA for combination group than tacrolimus monotherapy. 5
At the end of the study, after 12 months of treatment, the combination group (n=54) showed significantly better improvement in the primary end point, EOR with 66.7% of patients achieving greater than 50% of repigmentation as compared to only 39.2% in tacrolimus group (n=51). [Figure 1]
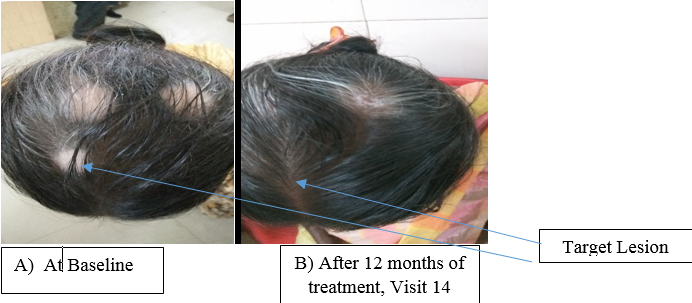
Significant improvement in grade of repigmentation (grade 6 response) noted in the vitiligo lesions at the end of study. [Figure 2]
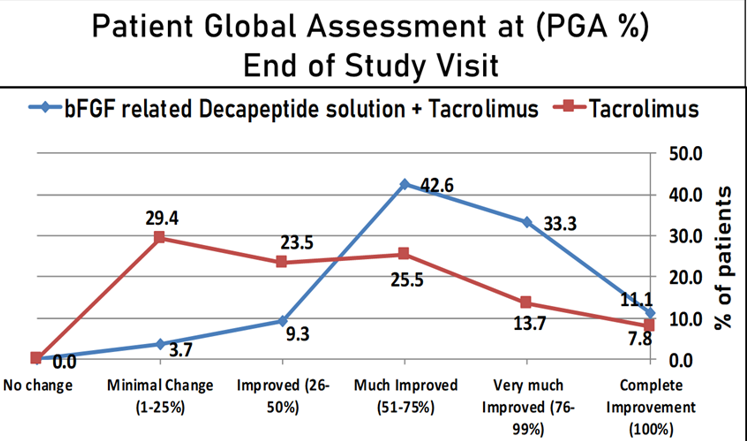
Moreover, complete repigmentation was achieved in the combination group among five patients (9.3%) as to none in tacrolimus group. The complete improvement in PGA was also noted in combination group among six patients (11.1%) in comparison with four patients (7.8%) from tacrolimus group. [Figure 3]
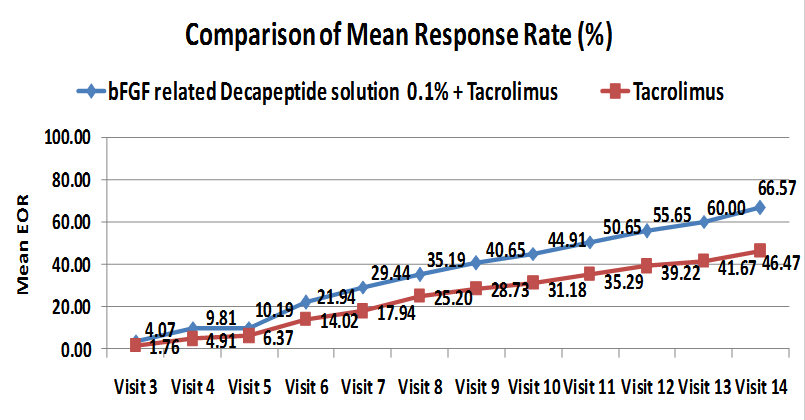
Significant increase in the mean response rates was seen among patients on combination therapy of bFGFrP solution 0.1% along with tacrolimus ointment from (4 months) visit 6 onwards. The mean response rate of bFGFrP solution 0.1% with tacrolimus ointment 0.1% was 21.94 % while that tacrolimus monotherapy was 14.02% at 4 months (visit 6). At the end of the study visit, significant mean response rate (66.75%) was observed with the combination therapy of bFGFrP solution 0.1% with tacrolimus 0.1% ointment compared to tacrolimus monotherapy response rate of 46.47%.
No adverse drug reactions were seen in both the groups at the end of the study.
Table 1
Graph 1
Extent of Repigmentation (EOR) > 50% at the end of 12 months of treatment was significantly greater in-patient receiving with bFGF related decapeptide 0.1% solution and Tacrolimus 0.1% ointment treatment (66.7% patients) versus Tacrolimus 0.1% ointment (39.2% patients)
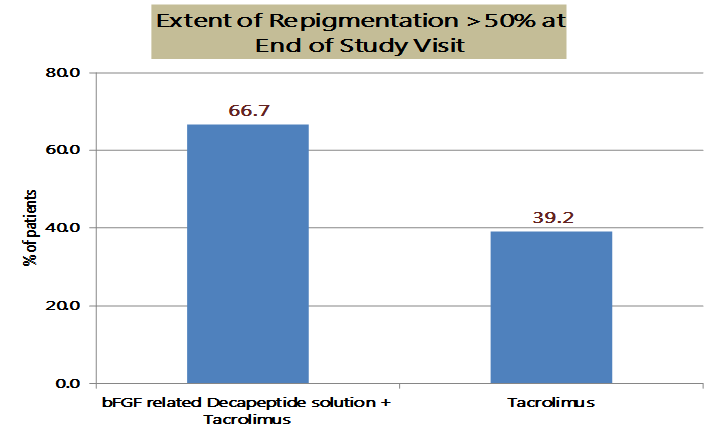
Figure 1
Grade 6 response following application of combination of bFGFrP 0.1% solution and Tacrolimus ointment.
Graph 2
Patient Global Assessment at the end of 12 months of treatment showed complete repigmentation (100% improvement) in 11.1% patients and Very much Improved (76 to 99% improvement) in 33.3% patients in-patient receiving combination treatment of bFG related decapeptide 0.1% solution and Tacrolimus 0.1% ointment
Discussion
The pathogenesis of vitiligo is complex and multifactorial; there is an accumulating evidence from various clinical studies about addition of different growth factors such as bFGF and increased re-pigmentation in vitiligo lesions.
No single treatment modality has been shown to have excellent results in vitiligo repigmentation by virtue of the interplay of many factors involved in the pathogenesis of disease. For excellent response rates, combining the treatment modalities supersedes the individual monotherapies.
Several studies and case reports on the use of topical immunomodulators in vitiligo have been published. Among immunomodulators, topical calcineurin inhibitors (TCIs) are often considered as mainstay of the topical treatments.
Among the topical immunomodulators, Tacrolimus is widely accepted drug for vitiligo treatment. Although it has shown great efficacy in face and neck vitiligo lesions but less effective for segmental types of vitiligo.6
The best results were obtained on lesions of the cephalic region, especially the face and neck, with tacrolimus 0.1% ointment twice daily. The subgroup meta-analysis done for these sites reveals, the marked response was seen only in 35.4% of patients.
Several combination strategies and novel approaches are increasingly used to achieve fast and marked repigmentation in this dynamic disease. In the current study, we compared the topical tacrolimus monotherapy with its combination with the bFGFRP. Our study demonstrated its safe and efficacious use as depicted in ample of published studies.
The published literature about the use of topical calcineurin inhibitors (TCIs) including tacrolimus for vitiligo often stated additional benefits of their use in combinations. In a systematic, review which evaluated the treatment responses of TCIs (tacrolimus-14 studies) monotherapy with the help of 36 studies (up to 2018) conducted in 941 patients. Marked response (≥75 percentage repigmentation) was noted only in 18.1% of 520 patients in 19 studies. While in children, the marked response was achieved only in 31.7% of patients in five studies.7
Another recent Systematic review of RCTs, evaluated total 19 studies including 814 patients to investigate the efficacy and safety of topical tacrolimus monotherapy in treatment of vitiligo. The trials were searched to October 2018 (2005-2018, published RCTs were included) also hints that combining tacrolimus to other treatment options like steroids, phototherapy and laser may be superior to using tacrolimus alone.8
Studies like Nada & Rashed et al.9 have also documented the role of bFGF in vitiligo. bFGF related Decapeptide is derived from bFGF which is a mitogen stimulating the growth as well as migration of melanocytes and therefore helps in repigmentation. There is interplay between the melanocyte growth factor deprivation theory and theory of autoimmunity. There is increased sensitivity of melanocytes to oxidative stress in vitiligo. Rationale for bFGFrP in treatment of vitiligo is based on the melanocyte growth factor deprivation theory and the clinical evidence generated in the Indian clinical trials (Abburi, & Samastha Laboratories, India, 2017) support bFGFrP as a drug for the management of vitiligo.10
In addition, mechanisms of repigmentation induced by photo-biomodulation therapy in vitiligo revealed He‐Ne laser irradiation significantly increase in basic fibroblast growth factor (bFGF) release from both keratinocytes and fibroblasts. Basic fibroblast factor is a putative melanocyte growth factor, which stimulates melanocyte migration.
Combination therapies in the management of vitiligo have been proven beneficial as they act via different mechanisms. Topical tacrolimus often has been co-prescribed with topical bFGFRP solution to the Indian patients with stable vitiligo.
The study demonstrated the benefits of combination of bFGFrP and Tacrolimus ointment in patients of stable vitiligo over the topical tacrolimus monotherapy with considerable safety after 12 months of treatment.
Limitations
There were few limitations in our study, although the study was randomized, it was an open label; a double blinded study with a larger sample size would have been of more value addition. A subgroup analysis as per the segmental and non-segmental types of vitiligo; the total duration of vitiligo as well as stability duration of vitiligo could have been done.
Conclusion
The study shows that after 12 months of treatment, the combination therapy with bFGFrP and Tacrolimus leads to significantly better and rapid repigmentation and response rates in stable vitiligo patients. The improvement in the primary end point, EOR was seen as early as 3-4 months. The duration of treatment, (12months) resulted in significantly higher complete repigmentation with considerable safety among patients in combination therapy group than tacrolimus therapy.
The combination therapy with bFGFrP and Tacrolimus achieves higher extent of repigmentation and response rates; better in terms of faster outcomes than tacrolimus monotherapy in treatment of stable vitiligo.