- Visibility 190 Views
- Downloads 16 Downloads
- DOI 10.18231/j.ijced.2020.051
-
CrossMark
- Citation
Melasma mystery: Oral tranexamic acid
- Author Details:
-
Uday Kr Udayan
-
Pankaj Kr Tiwary *
-
Ramawatar Singh
Introduction
Melasma is one of the most common cutaneous pigmentary disorder, frequently affecting people of asian ethnicity. Melasma is acquired localized hyper pigmentation of skin characterized by presence of asymptomatic light to dark brown colored macules on malar area, nose, forhead and sometimes chin. UV radiation,hormonal changes and genetic susceptibility have been proposed to be major etiological factors.
Despite availability of wide range of topical agents, melasma has been a consistant chalange for dermatologist as with topical therapy alone treatment response is often unsatisfactory and recurrences are very frequent. Adding on Chemical peels and laser treatments have affordability issue in developing countries. These all has lead to search of newer oral agents such as tranexamic acid, glutathione, polypodium leukotomas. Tranexemic acid seems most promising among new arrivals.
About the molecule
Tranexamic acid (trans 4 amino methyl cyclohexane carboxylic acid: TXA) has been known for its antifibrinolytic effect through the reversible blockade of lysine binding sites on plasminogen molecules. [1] TXA has been approved for treatment of menorrhagia in dosage of 2-4.5 g/day. [2] Its usage in the treatment of melasma was accidentally discovered and reported by Nijo in 1979. [3]
Aims and objective
This study was done to evaluate efficacy and safety of oral tranexamic acid in melasma.
Material and Methods
Prospective randomized controlled trial was conducted among 140 patients of clinically diagnosed moderate to severe melasma not responding to topical therapy alone. History were taken regarding any abnormal bleeding episode. Patients were investigated for bleeding time, clotting time, platelet count, those with abnormal results were excluded from the study. Patient’s age ranged from 18-51yrs (mean 31.4 ±8.2yrs). Dermascopic examination was done to assess type and extent of melasma as well as to document treatment response.
Patients were randomly divided into 2 groups, each consisting 70 patients. Group A patients were given oral tranexamic acid 250 mg BD along with SPF 40 sunscreen at daytime and hydroquinone 4% at night. Group B patients were given only topical treatment same as that of group A. Photographs were taken at baseline and follow-up. Treatment was continued for 4 months and further monthly follow up was done for another 4 months. Response was assessed on the basis of MASI score (malesma assessment severity index)[4] decrement, which was calculated at base line, 8th, 12th and 16th week. Patients were asked about any side effect experienced by them during course of treatment.
Results
Mean MASI score was 14.2 ± 3.8 (Group A) and 13.9 ±3.6 (group B) at start of the treatment
| Gender | Group A | Group B |
| Male | 19 | 21 |
| Female | 51 | 49 |
| Distribution | Group A | Group B |
| Frontal | 18 | 19 |
| Cenrtal- facial | 44 | 45 |
| Chin | 08 | 06 |
| Type of melasma | ||
| Epidermal | 23 | 21 |
| Mixed | 47 | 49 |
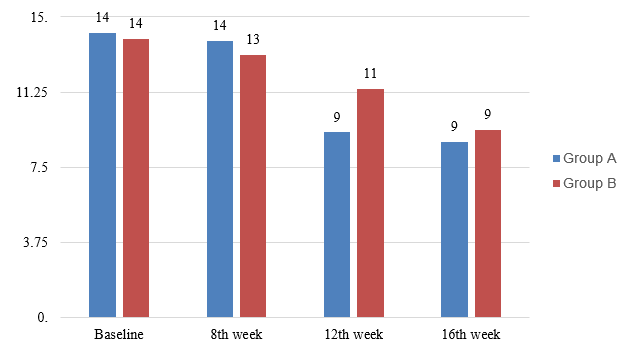
Among group A patients decrement in mean MASI score from baseline was significant at both 12th (9.24±2.8) and 16th week (8.76 ± 2.3) but not at 8th week (13.4±1.7), p value was <0.05 for both 12th and 16th month.([Figure 1])
In group B decrease in MASI score was statistically insignificant at 8th (13.1 ± 3.1) and 12th week (11.4 ± 3.5), p values being <0.05 for both. At 16 week we got significant response in group B (9.38±2.8) also but comparatively less than group A.
No significant side effects were observed in group A except mild gastric upset (2 patients) and menstrual irregularities (1 patient). None of the patients had bleeding episode or any other serious systemic abnormality. Among both groups.
4-5 patients had slight erythema and irritation at applied site.
2 Patients in group (A) left treatment in between including who suffered from oligo menorrhea. Among Group (B) 5 patients left treatment in between probably because of slower response and 3 of them were inclined towards getting treated with chemical peels and lasers.
In both groups response was better in epidermal type, less duration of disease and center- facial type of melasma. Forehead and chin sites were more difficult to treat.
At end of 8 months follow up period 4 patients in group (A) showed recurrence while in group (B) 6 patients had started developing pigmentation.
Discussion
In Melasma topical treatment is mainly directed at temporarily decreasing melanin synthesis by melanocytes while disease has multifactorial etiology which is only partially understood. Persistance of etiological factors leads to recurrence of lesion within short span of time resulting in patient’s dissatisfaction.
Further adding first line agent like hydroquinone has its own limitation. On pronged use it can cause ochronosis creating a resistant and helpless condition for dermatologists. Chemical peels and lasers have adjuvant role and have shown good response but results are not long lasting and affordability is major issue. Therefore effective new treatment modalities are always welcomed.
Tranexamic acid is a lysine anolog and posses antiplasmin activity. Plasmin has been detected to increase arachdonic acid (prostenoid precursor) synthesis and release by endothelial cell as well as ᾳ Melanocytic harmone activity. These two substance play important role in melanin synthesis by melanocytes, thus Tranexamic acid has shown valuable response in decresing melasma pigmentation.[5], [6], [7]
According to study done by Wu at al in 256 patients oral tranexamic acid had shown higher reponse (total improvement rate 80.9%) but thay had given TXA for longer period of time (6 months) and side effects were also minimal like our study.
In a study conducted by Karn D et al in Nepal among 260 patients, they had prescribed oral TXA for 3 months and follow up period was also less (3 months). Subjective patient satisfaction score was also used to evaluate response.
Oral Tranexamic acid has been successfully combined with IPL and Q switched NdYAG laser in a study conducted by Cho HH et al with no serious side effects.
In our study effiacacy and side effect profile of TXA corresponded with related studies except that we had higher number of recurrences during end of follow up period.
Conclusion
Tranexamic acid seems to be potential new medication as in various studies it has been shown to achieve melasma clearance, although durability of response is still questionable. Molecule is yet to be fully explored so that maximum effective and safe dose could be given according to an optimum regimen.
Source of Funding
None.
Conflict of Interest
None.
References
- C J Dunn, K L Goa. Tranexamic acid: a review of its use in surgery and other indications. Drugs 1999. [Google Scholar]
- K Wellington, A J Wagstaff. Tranexamic acid: a review of its use in the management of menorrhagia. Drugs 2003. [Google Scholar]
- T Nijo. Treatment of melasma with tranexamic acid. Clin Res 1979. [Google Scholar]
- C K Kimbrough-Green, Cem Griffiths, L J Finkel, T A Hamilton, S M Bulengo- Ransby. Topical retinoic acid (tretinoin) for melasma in black patients. Arch Dermatol 1994. [Google Scholar]
- N. Wang, L. Zhang, L. Miles, J. Hoover-Plow. Plasminogen regulates pro-opiomelanocortin processing. J Thromb Haemost 2004. [Google Scholar]
- W. C. Chang, G. Y. Shi, Y. H. Chow, L. C. Chang, J. S. Hau, M. T. Lin. Human plasmin induces a receptor-mediated arachidonate release coupled with G proteins in endothelial cells. Am J Physiol-Cell Physiol 1993. [Google Scholar]
- H Ando, M S Matsui, M Ichihashi. Addendum: Quasi-Drugs Developed in Japan for the Prevention or Treatment of Hyperpigmentary Disorders. Int. J. Mol. Sci. 2010, 11, 2566–2575. Int J Mol Sci 2010. [Google Scholar]
