Introduction
Dermatophytoses are among the commonest of dermatological infections occurring in India owing to the warm and humid climate, crowded living conditions and other socioeconomic factors. Even though dermatophytoses are not life-threatening, the consequences on DLQI cannot be overlooked. Within the past five years, there has been a dramatic yet unexplained surge in dermatophytoses in India. This is paralleled by the paucity of relevant literature on the same. Not only are we encountering unusual morphologies like pseudoimbricate and erythrodermic forms, the treatment aspect is also becoming a therapeutic menace. This changed face of dermatophytosis has resulted in prolonged duration of antifungal therapy, partial treatment response, and many times, treatment failure.1, 2, 3
Despite the availability of multiple antifungal agents, the management of dermatophytosis is still challenging.4 Currently in India, topical imidazoles and allylamines are the most commonly used antifungal drugs for the management of dermatophytosis.5 However, because of widespread use, the fungistatic nature of imidazoles, and concomitant use with topical corticosteroids, a rise in minimum inhibitory concentration (MIC) values and reduced response to these drugs have been reported.3 Hence there is a need for a different antifungal with a novel mechanism of action for the management of dermatophytosis.
Ciclopirox Olamine (CPO) is a hydroxypyridone derivative that differs in structure and mechanism of action from the other known antifungal agents.6 It acts through the chelation of polyvalent metal cations, such as ferric (Fe3+) and aluminium (Al3+), thereby causing inhibition of metal-dependent enzymes (cytochromes, catalase, and peroxidase) leading to the disruption of cellular activities such as mitochondrial electron transport processes, energy production, and nutrient intake across cell membranes. It also alters membrane permeability causing blockage of intracellular transport of precursors.6
Multiple clinical trials have shown the efficacy and safety of Ciclopirox Olamine in the management of dermatophytosis with overall cure rates ranging from 60 – 90%.7, 8 However, there are no studies comparing the effectiveness and safety of Ciclopirox to other antifungal agents, especially in the Indian setup.
Keeping this scenario in mind we conducted this study with the objective of comparing the effectiveness and safety of Ciclopirox Olamine 1% cream with that of Luliconazole 1% cream with background Itraconazole therapy in the management of dermatophytosis.
Materials and Methods
Study Design
This was an open-label, randomised, prospective, interventional, comparative, double-arm study conducted at two dermatology clinics in Mumbai and Ahmedabad. The study was carried out over a period of four months from May 2019 to August 2019. The study was approved by the institutional ethics committee. Written informed consent was obtained from all study participants. The study was conducted as per ICH GCP guidelines.
Patients Selection Criteria
Inclusion criteria
Following patients were included in the study
Both male and female patients aged ≥ 18 years and ≤ 60 years
Patients with multi-site tinea infection confirmed by positive KOH microscopy and culture (Multisite tinea infection was defined as the patients with lesions at more than two different anatomical sites on their body e.g. tinea corporis et cruris meaning patients with tinea lesions on his/her trunk and groin area. Only those patients were considered who were having involvement of 5 to 10% body surface area.)
Exclusion Criteria
Following patients were excluded from the study
Patients with negative 10% KOH microscopy and culture.
Pregnant or nursing females.
Patients with known hypersensitivity to the study drugs.
Patients with immunosuppressive disease or on immunosuppressive drugs.
5. Patients with liver dysfunction, a history of seizures, other clinically significant diseases, (e.g. cardiac, respiratory, gastrointestinal, renal disease).
Patients with history of drug or alcohol dependency or abuse.
Visits and follow-ups
At the initial visit, the patients were randomly allocated either to Ciclopirox Olamine 1% cream, twice a day, (Group I), or Luliconazole 1% cream, twice a day, (Group II), for six weeks. Both the groups received Capsule Itraconazole 100mg, twice daily, as a systemic antifungal along with anti-histamines for 4 weeks. After the initial visit, follow-up visits were carried out at weeks two, four and six. The treatment was then discontinued, and patients were followed up clinically till week eight to assess any worsening of symptoms. During each visit, patients were evaluated for the improvement of their symptoms subjectively, while a simultaneous objective assessment was done in terms of erythema, itching, and scaling. 10% KOH examination and culture was performed at the initial visit, and at the end of six weeks.
Effectiveness Assessment
Primary effectiveness endpoint
The primary effectiveness endpoint was the percentage of patients achieving complete cure at the end of the treatment period from baseline. Complete cure was defined as the patients achieving both clinical cure and mycological cure at the end of the treatment.
Secondary effectiveness endpoint
The secondary effectiveness endpoints were:
The percentage of patients achieving clinical cure at the end of the treatment period. Clinical cure was defined as clear or almost clear symptoms [scaling, erythema, and pruritus] at the end of the treatment.
The percentage of patients achieving mycological cure at the end of the treatment period. Mycological cure was defined as negative microscopy under potassium hydroxide (KOH) examination and negative culture at the end of the therapy.
Improvement in total symptom score from baseline at the end of the therapy. The total symptom score was the average of scores of all the signs and symptoms.
For effectiveness assessment, each patient was evaluated for clinical and mycological improvement. Clinical improvement was assessed by evaluating the improvement in the common symptoms of dermatophytosis (scaling, erythema, and itching) on a five-point scale (0 – 4) with 0 being the complete resolution of symptoms and 4 being the most severe symptom. Mycological cure was determined by the results obtained by KOH mount and fungal culture in Sabouraud dextrose chloramphenicol agar medium followed by microscopic examination.
Safety Assessment
Safety assessment was done by analysing all the AEs reported by the patients during the treatment and monitoring of liver enzymes. Since itraconazole therapy was prescribed for a period of 4 weeks, liver function tests were done at baseline, at week 2 and at week 4 to assess the effect of itraconazole on liver function of patients.
Data analysis
Descriptive statistics were used to summarise effectiveness and safety endpoints using GraphPad Prism version 8 (San Diego, California: GraphPad Software Inc., 20057). Quantitative variables were analysed using mean and standard deviation, while categorical variables were analysed using frequencies and percentages. P values ≤0.05 were considered statistically significant.
Results
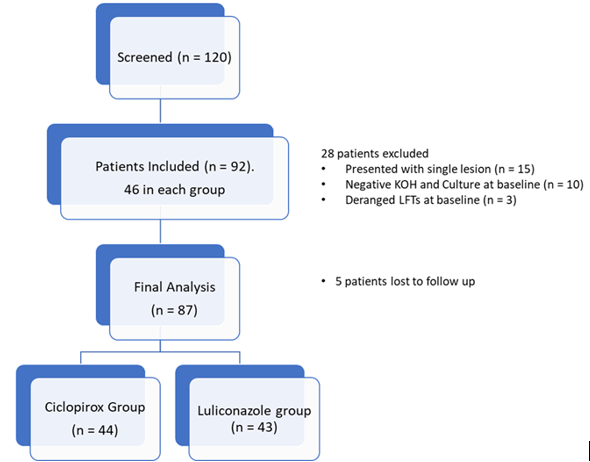
Among 120 patients screened, 92 patients who fulfilled the inclusion criteria were included in the study, with 46 patients in each group. Forty-four patients in the Ciclopirox group and fourty three patients in the Luliconazole group completed the follow-up period of eight weeks and were considered for the final analysis. Data from seven patients was excluded from the final analysis. Five patients were lost to follow-up. (Figure 1)
The average age of the patients was 38.28±11.72 years in the Ciclopirox group, while it was 35.72±13.18 years in the Luliconazole group. Out of 47 patients in the Ciclopirox group, there were 21 males and 26 females; while in the Luliconazole group, there were 21 males and 25 females. A majority of the patients were diagnosed with tinea corporis et cruris in the study groups, followed by tinea corporis et cruris et faciei; cruris et corporis et barbie and tinea mannum et cruris et corporis. There was no statistical difference (p > 0.05) between both the groups at baseline with respect to age, sex, diagnosis, KOH and culture positivity. The demographic characteristics of the patients are described in Table 1.
Table 1
Baseline Demographic Details
Effectiveness Analysis
Complete Cure
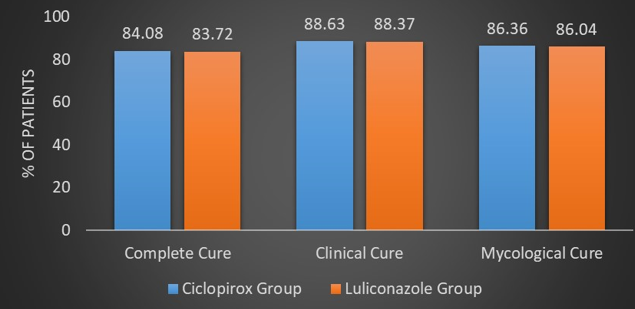
Out of 44 patients in the Ciclopirox group, 84.09% (n = 37) patients achieved a complete cure, while out of 43patients in the Luliconazole group, 83.72% (n = 36) patients achieved a complete cure. There was no statistical difference between the two groups with regards to the primary endpoint of a complete cure (p = 0.9625). (Figure 2, Figure 3, Figure 4) (Table 2)
Table 2
Effectiveness assessment of patients inCiclopirox and Luliconazole group
|
|
Ciclopirox Group |
Luliconazole Group |
p value |
|
Complete Cure |
84.08 |
83.72 |
p = 0.96 |
|
Mycological Cure |
86.36 |
86.04 |
p = 0.96 |
|
Clinical Cure |
88.63 |
88.37 |
p = 0.96 |
Figure 3
Improvement in lesions with Itraconazole + Ciclopirox Combination; A, C- Extensive tinea corporiset cruris at baseline; B, D- Complete resolution of symptoms after 6 weeks of therapy
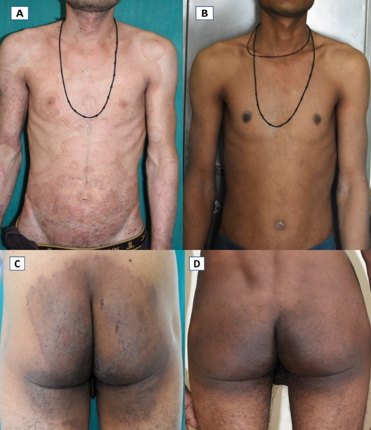
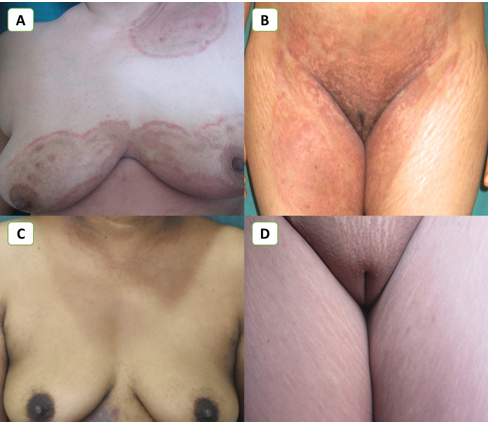
Figure 4
Improvement in lesions with Itraconazole + Luliconazole Combination; A, B- Extensive tinea corporiset cruris at baseline; C, D- Complete resolution of symptoms after 6 weeks of therapy
Clinical Cure
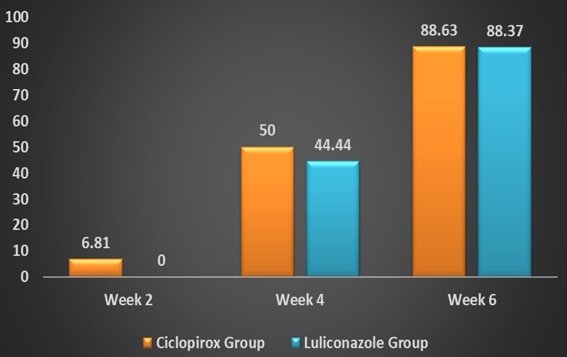
After six weeks of antifungal therapy, 88.63% (n = 39) patients and 88.37% (n = 38), patients achieved a clinical cure, i.e. a complete resolution of their symptoms in the Ciclopirox group and the Luliconazole group respectively. (Figure 2) Three patients (6.81%) achieved clinical cure in the Ciclopirox group compared to 0 in the Luliconazole group at week two. Similarly, numerically more patients achieved clinical cure in the Ciclopirox group compared to the Luliconazole group at week four (50% vs. 44.44%). (Figure 5) (Table 2)
Mycological Cure
Forty four patients in each group were KOH and culture positive at baseline. Out of 44 patients in the Ciclopirox group, 86.36% (n = 38) patients achieved a mycological cure, while out of 43 patients in the Luliconazole group, 86.04% (n = 37) patients achieved mycological cure, i.e. negative microscopy potassium hydroxide (KOH) examination and negative culture. (Figure 2) There was no statistical difference between the two groups with regard to clinical cure and mycological cure at week six. (p > 0.05) (Table 2)
Total Symptom Score (TSS)
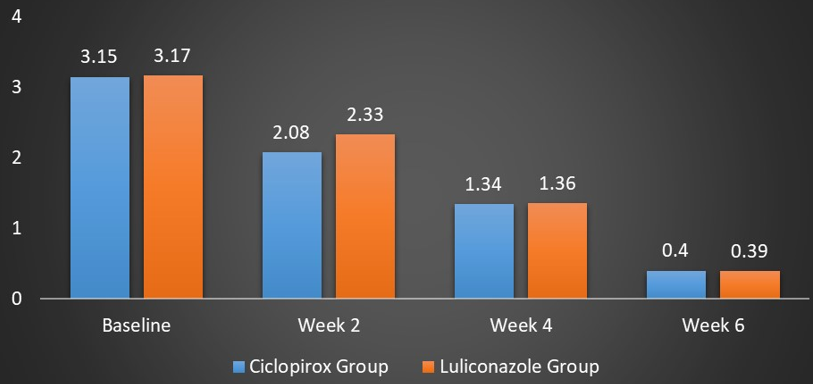
The mean TSS at baseline in the Ciclopirox group was 3.15 ± 0.54 while in the Luliconazole group was 3.17 ± 0.32. There was significant reduction in TSS during each follow-up visit in both the groups compared to the baseline. After two weeks, there was statistically significant improvement in TSS in the Ciclopirox group compared to the Luliconazole group (2.08 ± 0.4 vs. 2.33 ± 0.3; p = 0.02). However, at week four and week six, no such statistical difference was observed between the two groups with p > 0.05. At week four, the mean TSS was 1.34 ± 0.46 and 1.36 ± 0.5 in the Ciclopirox group and the Luliconazole group respectively (p = 0.13). After six weeks, the mean TSS in the Ciclopirox group was 0.40 ± 0.6 while in the Luliconazole group, it was 0.39 ± 0.7 (p= 0.65). (Figure 6) (Table 3).
Safety assessment
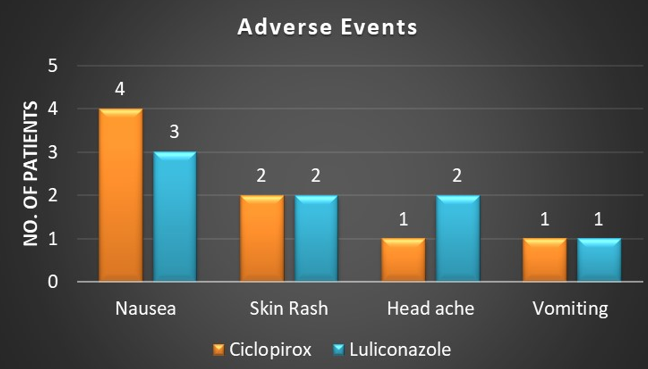
9.09% (n = 4) patients in the Ciclopirox group and 9.30% (n = 4) in the Luliconazole group reported one or more drug related AE. Nausea was the most common side effect reported by patients in both the groups followed by skin rash at application site, vomiting andheadache.. Adverse events like nausea, vomiting and headache were because of itraconazole. All the AEs were mild in nature and resolved during the study; none of the patients discontinued the therapy because of AEs. (Figure 7)
Liver function of all the patient was evaluated by analyzing the levels of Serum glutamic oxaloacetic transaminase (SGOT) and Serum glutamic pyruvic transaminase (SGPT) before initiation of itraconazole at baseline, at week 2 and at week 4 after completion of itraconazole therapy. The baseline SGOT & SGPT levels were 19.97 ± 7.34 and 19.86 ± 11.40 respectively. After 2 weeks of itraconazole therapy there was no significant change in SGOT and SGPT levels (19.06 ± 7.44 and 18.62 ± 10.70 respectively, p = 0.1). At week 4 similar results were seen with no significant change in SGOT and SGPT levels (19.76 ± 7.40 and 19.02 ± 11.50 respectively, p = 0.1).
Discussion
Dermatophytic infections are one of the commonest skin infections in India, with an increase in the incidence of difficult-to-treat cases. 1, 2, 3 Despite the availability of multiple antifungal agents, increasing MIC values to commonly used antifungal agents, and the misuse of topical steroids has led to distressing relapses in patients in recent times. 4 With a lack of recent studies on the efficacy of antifungals in the current scenario, dermatologists are using hit-and-trial methods, since the results of studies of antifungal effectiveness conducted many years ago cannot be extrapolated to the current scenario. 1, 2, 3, 4 We evaluated the efficacy and safety of the recently launched novel antifungal Ciclopirox Olamine 1% and the most commonly used topical antifungal Luliconazole 1% with background systemic antifungal therapy in the management of dermatophytosis.
In our study, the complete cure rate was achieved by 84.09% patients in the Ciclopirox group, while by 83.72% patients in the Luliconazole group with background systemic antifungal therapy of Itraconazole 100mg twice daily. 86.36% and 86.04% patients in our study achieved mycological cure rate in ciclopirox and luliconazole group respectively. Similarly, 88.63% and 88.37% patients achieved clinical cure in ciclopirox and luliconazole group respectively. There are no previous clinical studies which evaluated the clinical efficacy of combination of topical ciclopirox or luliconazole with oral itraconazole in management of dermatophytosis. Howevere multiple previous studies by various authors justifies the results achieved in our studies.
Earlier studies by Bagatell et al., Kligman et al., Cullen et al., Corte et al. have shown variable but appreciable complete cure rates for dermatophytosis in the range of 60 – 90% following Ciclopirox therapy. 9, 10, 11, 12 Similarly various studies by Watanabe et al., Jerajani et al., Selvan et al. have reported a complete cure rate in the range of 80 – 90% following the Luliconazole therapy. 13, 14, 15 Mycological and clinical cure rates reported by these authors for ciclopirox and luliconazole were in the range of 70 – 90%. 9, 10, 11, 12, 13, 14, 15 Similarly various earlier studies have shown variable but appreciable cure rates for dermatophytosis in the range of 72.7 – 96.6% following itraconazole therapy. 16, 17, 18
There was no statistically significant difference between the two groups with regard to complete cure rate, mycological cure rate and clinical cure rate at the end of therapy. Numerically more patients in the Ciclopirox group did achieve early clinical cure at week two and week four compared to the Luliconazole group. This could be attributed to the synergistic activity of Ciclopirox in combination with itraconazole because of the different mechanisms of action, 19, 20 whereas Luliconazole has a mechanism of action similar to that of itraconazole, thus minimising the chances of synergy.
In the present study, both the drugs were associated with significant improvement in signs and symptoms of patients with dermatophytosis from the second week onwards. Patients in Ciclopirox group showed faster improvement in signs and symptoms compared to patients in luliconazole group. This could be attributed to its potent anti-inflammatory, fungicidal action and synergistic activity with itraconazole.
In our study, amongst 87 patients who were culture positive at baseline, in both groups, T. Mentagrophyte was the dominant species with a prevalence of 67.81% (n = 59/87) followed by T. Rubrum 21.83% (n = 19/87). These results are also in accordance with recent epidemiological studies conducted in India by Mahajan et al., Nenoff et al., and Bhatia et al. which reported the increasing trend of T. Mentagrophytes in India in recent times. 21, 22, 23
With regard to safety, only 9.09% patients in the Ciclopirox group and 9.30% patients in the Luliconazole group reported one or more AE in our study. All the adverse effects were of mild to moderate severity and none of the patients required discontinuation of the therapy. These results are in accordance with the safety profiles of both Ciclopirox and Luliconazole reported in earlier studies. 9, 10, 11, 12, 13, 14, 15 Adherence to treatment was excellent in all the patients.
Conclusion
To our knowledge, this is the first Indian study comparing the efficacy and safety of Ciclopirox cream 1% to Luliconazole cream 1 % with background itraconazole therapy. The results of this study prove that Ciclopirox is efficacious and safe in the management of dermatophytosis. This study also proves that Ciclopirox is comparable to Luliconazole with respect to mycological and complete cure rate. The study also highlights the earlier relief of signs and symptoms in patients with dermatophytosis with the use of a combination of two different classes of antifungal drugs, as compared to a combination of antifungal drugs from the same class. In conclusion, the results of the study show that Ciclopirox is a novel antifungal, which can be instrumental in the management of dermatophytosis in the current scenario in India.
Acknowledgments
The authors are thankful to Glenmark Pharmaceuticals for donating the trial medications free of cost to the researchers. The authors are also grateful to the faculty, residents and staff of the Department of Dermatology for their cooperation during the entire duration of the study.