Introduction
Leprosy, also recognized as Hansen’s disease, is a chronic granulomatous infectious disease caused by Mycobacterium leprae. It is an ancient disease in India with its early description in ‘Sushruta Samhita’ written in 600 BC.1
Mycobacterium leprae is an acid-fast, gram positive bacilli having special affinity for Schwann cell of nerve. It was first discovered by Norwegian physician Gerhard Henrik Armaeur Hansen in 1873. 2 This obligate intracellular parasite has a life span of 6 months and generation time of 12-14 days.3 Leprosy is a systemic disease affecting skin, peripheral nerves muscles, eyes, bones, testes and other internal organs.
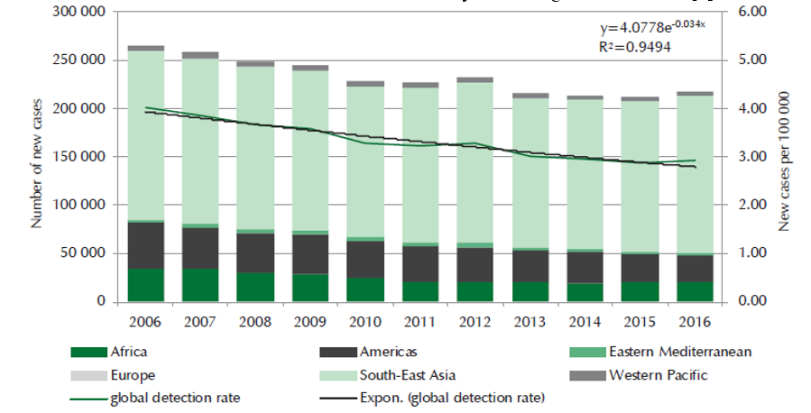
Diagnosis of leprosy is many times clinical but in doubtful cases, histopathological studies are supportive and confirmatory. The present day applications of histopathology are many folds and include diagnosis of reaction states and relapse, accurate classification and assessment of response to chemotherapy and / or immunotherapy. Histopathological study of leprosy is very important in understanding the disease, its various manifestations and complications. Currently around 126,164 new cases are detected annually (Year 2017-18) with PR 0.67 per 10,000 population and ANCDR (Annual New Case Detection Rate) 9.27 per lakh population (Figure 1). More than 85% of global burden is currently seen in the following seven countries and India contributes to around 60% of the burden.4(Table 1)
Table 1
Number of leprosy patient in seven countries 4
|
Country |
Number |
|
India |
1,26,164 |
|
Brazil |
26,875 |
|
Indonesia |
15,910 |
|
Bangladesh |
3,754 |
|
Democratic Republic of Congo |
3,649 |
|
Nepal |
3,215 |
|
Ethiopia |
3,114 |
As per National Leprosy Eradication Programme annual new case detected during 2016-17 was 7545 and prevalence rate per 10,000 as on March 2017 was 0.78 in Madhya Pradesh and annual new case detected during 2016-17 was 98 and prevalence rate per 10,000 as on March 2017 was 0.30 in district Ujjain.6
Social stigma arising out of fear, ignorance and superstitious beliefs continue to be a major stumbling block in leprosy control measures. Another added cause are appearance of drug resistant strains of M.leprae problem of microbial persistence and inadequate and untimely treatment.
Hansen’s disease was eliminated from India in terms of statistical prevalence but from disease problem point of view it still poses many challenges. In unavailability of vertical program active case finding is not there. The medical facility at the peripheral level must be equipped to spot leprosy and to arrest the transmission and disability. Here comes the importance of this study carried out in a tertiary care center situated in Ujjain, Madhya Pradesh serving mainly rural population.
Materials and Methods
This was a Tertiary hospital based observational study of new Leprosy patients in general population who were clinically diagnosed to have Leprosy in the outpatient department of dermatology, R. D. Gardi Medical College and C. R. Gardi Hospital, Ujjain (M.P.) This study was carried out in a time period of 1 year extending from January 2019 to December 2019.
Exclusion criteria
Patients who were not cooperative or not willing to participate in the study.
All old diagnosed leprosy cases already on treatment.
Patients with terminal illness.
Total sixty (60) patients who were clinically with diagnosed leprosy were chosen after taking a written consent. A detailed history of age, gender, occupation and socioeconomic status was taken and detailed general examination was carried out in all the patients. Local examination of skin lesions was carried out. All the peripheral nerves were palpated for enlargement.
All routine investigations as well as Special investigation like Slit-Skin Smear and Biopsy were done. The findings were recorded in the proforma and tabulated in the master chart. The results were analyzed and discussed in detail.
Results
Patients were diagnosed and classified on the basis of Ridley and Jopling Classification.
The mean age of the patients was 42.95 years with a range of 12 to 80 years. Maximum number of patients belonged to age group of 21-40 years. Childhood cases (≤ 15 years) were only 2 in number. The study included 45 males and 15 females with male female ratio of 3:1.
Table 2
Distribution of patients according to their socio-demographic characteristics.
Positive family history of Leprosy was found only in 3 patients (5.0%). Illiterate (35 in number), Hindus (93.3%), Rural inhabitants (93.3%), Married individuals (88.3%), Patients from lower socio-economic status (58.3%) and semi-skilled worker formed the bulk of the patients enrolled (Table 2).
Clinically MB (Multibacillary) cases outnumbered PB (Paucibacillary) cases by a ratio of 1.75: 1. Borderline tuberculoid (BT) was the commonest clinical spectrum reported (19 patients).
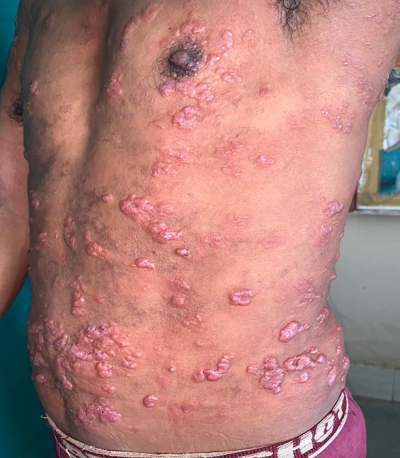
Lesions most commonly presented in form of Patches (50 patient) & Plaques (38 patients) mostly distributed on extremities (upper limb+ lower limbs) and more commonly in asymmetrical fashion (90%). Majority of clinical lesion were anesthetic (53.3%), presented with hypopigmentation (83.3%).
On Neurological examination, Ulnar Nerve was the commonest to be involved followed by lateral Popliteal Nerve. Pattern of nerve involvement was predominantly multiple asymmetrical (50 patients) and tingling, numbness and sensory loss (65%) were chief neurological complaints elicited. Proportion of Hand and foot deformity were also significant in 10 patients with ulcers being the most common issue (11.6%).
Only 9 patients developed Lepra Reaction. Type I reaction in 2 patients & Type II reaction in 7 patients respectively. Patients developing Type I reaction belonged to Borderline tuberculoid (BT) and that of Type II reaction in Lepromatous (LL) subtype.
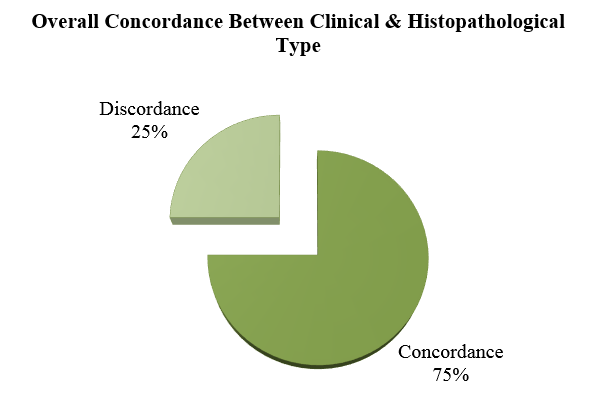
In clinical and histopathological correlation, overall parity of (75%) was achieved with maximum concordance seen in two polar types like Tuberculoid (TT) (100%) & Lepromatous (LL) (76.9%) followed by Borderline Lepromatous(BL) (70.5%), BT (63.15%). Least parity was noted in Borderline Borderline (BB) (25%). (Table 3)
Table 3
Clinico-Histopathological Correlation
(TT =Tuberculoid, BT =Borderline Tuberculoid, BB=Mid-Borderline,
BL=Borderline Lepromatous, LL=Lepromatous, IL=Indeterminate Leprosy)
Maximum correlation was seen with TT (100.0%) followed by LL (76.90%), BL (70.50%), BT (63.15%) & BB (25%). (Figure 2)
Discussion
In the present study, age of the patients ranged from 12-80 years with maximum number of patients in age group of 21-40. Age distribution of the present study was comparable with other studies like Kautuk K. P et al (2020), 7 Mahajan R et al (2018). 8 This could be due to more chances of exposure, opportunities for infection and increased awareness regarding seeking medical advice in this age group.
Globally, proportion of new child cases is 8.8% (WHO, globally leprosy update 2015). 9 It is an indicator of active transmission of disease in the community. In our study, childhood leprosy (≤ 15 years of age) were found in only 2 patients (3.3%). This proportion is lower than reported by Chhabra et al (2015) (9.3%)10 and by Singal et al (2011)(9.6%). 11
Table 4
Comparison with Gender
|
Author |
Male (%) |
Female (%) |
Total (%) |
M:F Ratio |
|
Present study |
75 |
25 |
100 |
3:01 |
|
Kautuk K. P et al (2020) 7 |
69.3 |
30.7 |
100 |
2.3:1 |
|
Verma A et al (2020) 12 |
64.6 |
35.4 |
100 |
- |
|
Mahajan R et al (2018) 8 |
68.69 |
31.31 |
100 |
2.2:1. |
|
Mehta B et al(2012) 13 |
- |
- |
100 |
2.3:1 |
|
Jayalaxmi et al (1980) 14 |
73.68 |
26.31 |
100 |
2.8:1 |
Male to female ratio was 3:1 which shows males comprised the majority of our patients (Table 4). Similar gender ratio is also demonstrable in other studies shown above. Although male gender bias has been associated with Leprosy since the Sulfone era, much greater male dominance over female might be due to their greater mobility & increased access to health facilities.
In the present study, majority of the patients were illiterate, semi-skilled by occupation and belonged to lower socio economic status. The results were comparable with other studies Verma A et al (2020), 12 Doshi et al (2016)15 Mehta B et al (2012).13 Educational status of leprosy cases emphasizes on the unawareness and lack of information among common people about leprosy. The socioeconomic status reflects that leprosy is a disease of the poor and low socio-economic strata person surviving in over-crowding home condition.
In the study, 73.3% patients belonged to rural areas. Though a similar kind of study from Maharashtra concluded by Doshi et al (2016) 15 that new case detection rate and prevalence of leprosy were greater in urbans areas. This disparity can be explained by the fact that our hospital drains a lot of rural population from western M.P and Rajasthan border areas.
Positive Family history could be elicited in 5.0% of the cases in the present study. This observation is slightly on the lower side as compared to observations of other studies Mahajan R et al (2018), 8 Mehta B et al(2012), 13 Salodkar AD et al (1995). 16 This stresses importance of family contact as a source of infection where the source and susceptible individual are close to each other.
In the present study, majority of the patients (75.0%) presented with their complaints within 2yrs. The results were comparable with other studies Nigam P et al (1977).17
72% of our patients had more than one lesion, which is not in conformity with the previously published studies by Selvasekar et al.1999.18 This can be explained as people may ignore a single lesion and might not come to tertiary hospital for such a minor ailment.
The morphology of the lesions was varied in different studies like by Ganpati et al, (1984).19 Hypopigmented plaques followed by patches were the most common type of lesions in our patients. The distribution of leprosy lesions also varied in different studies as shown by Selvasekar et al, (1999).18 The majority of the patients (84.0%) in the present study had lesions on extremities, which was in agreement with Chaudhary RC et al (1981).20
In the present study, deformities were present in 20.0% of patients. A higher occurrence of deformities were noted by Kautuk K. P et al7 (50%), Mahajan R et al 8 (40.11%), Mehta B et al13 (53.33%) and Jindal N et al21 (54.47%).
Ocular features were noted in 13.4% of patient in the present study which was higher than that of Jindal et al21 and lower than that of Mahajan R et al.8 Tegta et al22 noted eye involvement in 8.6% patients with conjunctivitis being the most common as in our study.
In the present study, BT (19 patients) was the commonest clinical spectrum as in other studies also (Tekwani D et al 23 2017, Mehta B et al 13 2012, Shivswamy KN et al 24 2012, Sharma A et al 25 2008, Bhushan et al 26 2000). In our study, the proportion of MB cases were 63.33% and PB cases were 37.67%. Similar findings were shown by study done by Kurup et al 27 (2018) also where MB& PB cases were 71.9% & 26.6% respectively.(Table 5)
Table 5
Comparison with Clinical Diagnosis
|
Clinical Diagnosis |
TT |
BT |
BB |
BL |
LL |
IL |
Total |
|
Present Study (%) |
5.0 |
31.7 |
13.3 |
28.3 |
21.7 |
- |
100 |
|
Singh P et al (2019) 28 |
15 |
19 |
- |
12.5 |
22.5 |
2.5 |
100 |
|
Tekwani D et al (2017) 23 |
8.88 |
62.2 |
2.96 |
17.77 |
5.92 |
- |
100 |
|
Mehta B et al (2012) 13 |
24 |
29 |
6 |
21 |
20 |
- |
100 |
|
Shivswamy KN et al (20l2) 24 |
17.5 |
38.4 |
2.7 |
13.1 |
12.6 |
15.7 |
100 |
|
Sharma A et al (2008) 25 |
7.7 |
33.6 |
33.6 |
6.9 |
11.7 |
7.5 |
100 |
|
Bhushan et al (2000) 26 |
8.2 |
78.7 |
- |
8.2 |
4.9 |
- |
100 |
|
Jayalaxmi et al (1980) 14 |
25.0 |
38.16 |
- |
11.84 |
25 |
- |
100 |
Multibacillary leprosy (MB) cases are clinically important as they are a major reservoir of infection and also predisposed to reactions and subsequent deformities. The greater proportion of MB cases in our study is probably due to fact that our hospital caters to the very under privileged section of society in western M.P.
Lepra reactions were noted in 15% patients of the study with type-II reaction more than 3 times common than type-I reaction. Almost similar findings regarding Lepra reaction were given by Salodkar et al where 11% cases presented with type-II reactions and bing 4 times more frequent than type-I reaction. High proportion of reactions might be attributed to the fact that many patients seek medical advice only when they develop reactions and our study included a good number of LL cases 13 as well.
Table 6
Comparison of Slit Skin Smear
|
Author |
AFB Present (%) |
AFB Absent (%) |
Total Cases |
|
Present study |
60 |
40 |
100 |
|
Mahajan R et al (2018) 8 |
54.01 |
45.89 |
100 |
|
Mehta B et al (2012) 13 |
88.89 |
11.11 |
100 |
|
Ganpati R et al (1984) 19 |
30.7 |
69.3 |
100 |
|
Jayalaxmi et al (1980) 14 |
44.73 |
55.27 |
100 |
In the present study, 60% cases showed acid-fast bacilli (AFB) on slit skin smear (Table 6). The difference in other studies might relate to difference in the clinical presentation of the disease at the time of study. This stresses on importance of carrying out bacteriological index especially in the borderline group, which shows a continuous shift in the immunological spectrum.
Table 7
Comparison with parity
|
Authors |
TT |
BT |
BB |
BL |
LL |
|
Present Study |
100 |
63.15 |
25 |
70 |
79.90 |
|
Singh P et al (2019) 28 |
100 |
68.42 |
00 |
100 |
88.88 |
|
Tekwani D et al (2017) 23 |
83.33 |
79.76 |
54.16 |
50 |
25 |
|
Shivswamy KN et al (20l2) 24 |
73.3 |
64.1 |
50 |
73.3 |
84.2 |
|
Mehta B et al (2012) 13 |
75 |
58 |
33.33 |
71.4 |
90 |
|
Mathur MC et al (2011) 29 |
73.2 |
89.74 |
64.7 |
72.4 |
95.2 |
|
Sharma A et al (2008) 25 |
47.37 |
53.01 |
37.35 |
58.82 |
75.86 |
|
Moorthy BN et al (200l) 30 |
46.15 |
66.35 |
50 |
70 |
80 |
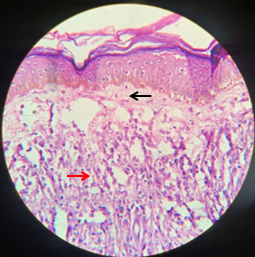
Leprosy presents with different clinico-histopathological forms which depends on immune status of the host. One of the major aims of this study was to correlate clinical diagnosis of new leprosy cases with the histopathological diagnosis of skin biopsies stained with Haematoxylin and Eosin followed by Fite-Faraco stain.
In the present study, maximum parity i.e. 100% was observed in tuberculoid leprosy, followed by lepromatous leprosy (76.90%) and minimum in Borderline group (BT+ BB+ BL) of leprosy (Table 7). Similar less agreement in Border line spectrum was shown by studies of Singhi et al31 (2003), Bhatia et al, 32 Shenoi & Siddappa.33 Parity in the polar group is maximum because they are stable and show a fixed histopathology, while Borderline groups (BT+BB+BL) are in a continuously changing immunological spectrum and may have different histopathology in different site and lesion.
Table 8
Comparison of overall Clinico-Histopalhological Correlation
|
Authors |
Overall clinico- Histopathological correlation |
|
Present study |
75.0% |
|
Singh P et al (2019) 28 |
82.5% |
|
Tekwani D et al (2017) 23 |
72.59% |
|
Sharma A et al (2008) 25 |
53.44% |
|
Moorthy BN et al (200l) 30 |
62.63% |
|
Bhushan et al (2000) 26 |
60.60% |
|
Ridley & jopling et al (1966) 34 |
68.30% |
In the present study of 60 newly diagnosed cases of Hansen's disease, overall parity observed was 75.0% (Table 8). This was intermediate with in the results of other studies. The difference in clinical and histopathological diagnosis may relate to size & site of the biopsy, age of the lesion and immunological status of the patient at the time of taking biopsy. Serial biopsies from the same lesion or from paired lesions is advisable for more accurate histo-pathological correlation.
Figure 1
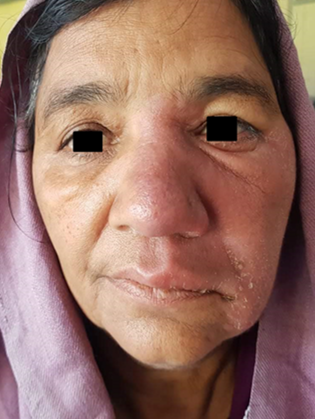
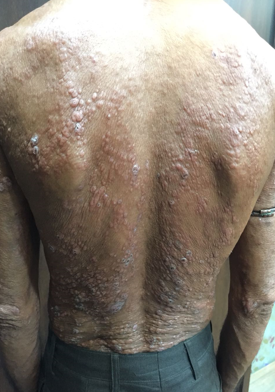
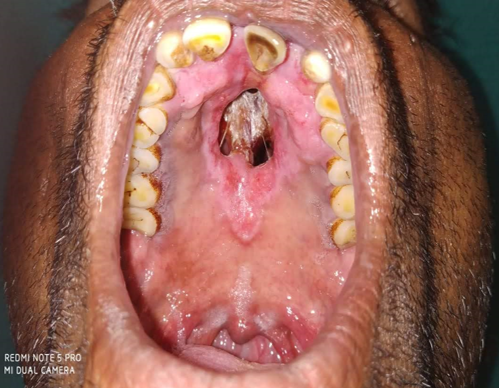
Infiltrated skin lesions, Saddle nose in a 14 year old child presenting with LL polar variant

Conclusions
In spite of decline in leprosy cases at national level, it surely continues to be a health concern. Predominance of MB cases and finding of disease in rural population in this study emphasizes the need of spread of awareness about the disease, facilities for investigation and early diagnosis and unhindered provisions of therapy to prevent deformities. Although this study was a retrospective tertiary hospital based, still it gives a general picture about the current trends of Hansen’s disease in this particular region. The present study highlight the importance of both clinical & histopathological examination and determining parity between their findings and we hereby support the theory that in case of confirmed discrepancy the more advanced findings must be given more priority and the patient to be classified and managed accordingly.