Introduction
Hair is an appendage of skin that has attracted more attention for its cosmesis than its medical relevance. Androgenic alopecia (AGA) is a genetically determined autosomal dominant,1 polygenic, inherited from either parent, androgen-dependent disorder of hair. Polygenic factors play a significant role in modifying the age of onset, pattern of loss and rate of progression of male pattern baldness (MPB).Gene association studies have now identified an association of MPB with a polymorphism of the androgen receptor gene on the X chromosome. So far this is considered to be the most common cause of hair loss in men and women. It is characterised by progressive decline in the duration of anagen, an increase in the duration of telogen and miniaturization of scalp hair follicles. Androgenetic alopecia usually presents as non-scarring hair loss under the influence of androgens (dihydrotestosterone, DHT); the androgen susceptibility of the follicles plays an important role.2
Current strategies for treatment of hair loss are mainly focussed on promoting cellular proliferation and differentiation during hair growth cycle. FDA approved drug therapy include Finasteride and Minoxidil.3, 4, 5 Minoxidil can prolong the anagen phase and to promote survival of dermal papilla cells and increase in hair follicle size.6, 7 Important actions of minoxidil on the hair follicles include - increased expression of vascular endothelial growth factor (VEGF) mRNA in the dermal papillae, activation of cytoprotective prostaglandin synthase-1, an enzyme that stimulates hair growth and increased expression of hepatocyte growth factor (HGF) m-RNA which is another hair growth promoter and vasodilatory effects.
Platelet Rich Plasma (PRP) represents an autologous concentration of human platelets in a small volume of plasma having 4-7 times the platelet concentration above the normal blood.8 The basic principle behind PRP injection is to deliver high concentrations of growth factors like9 VEGF (vasoendothelial growth factor), EGF(epidermal growth factor),FGF(fibroblast growth factor), IGF(insulin-like growth factor), and TGF(transforming growth factor) to the scalp, for the purpose of stimulating hair regrowth. Growth factors appear to act in the bulge area of the follicle, where they bind to their respective receptors located in stem cells. In the bulge area, primitive stem cells of ectodermal origin are found and in matrix, germinative cells of mesenchymal origin are found. Interactions between these two kinds of cells as well as with binding growth factors (PDGF, TGFβand VΕGF) activate the proliferative phase of the hair, giving rise to the future follicular unit.10
Aims and Objectives
To compare efficacy of Topical 5% Minoxidil alone versus Topical 5% Minoxidil with Autologous Platelet Rich Plasma (PRP) therapy in patients with Androgenetic Alopecia.
Materials and Methods
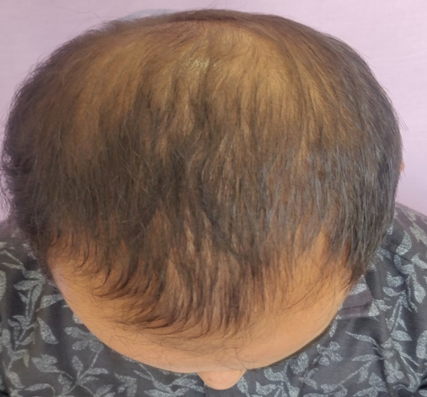
This is a Prospective, comparative study which was done in Department of Dermatology GMERS Medical College, Gandhinagar a tertiary care hospital in Gujarat during September 2018 to May 2020. In this study sample size was 62 with 31 in each group. After obtaining consent from the patients, adult males diagnosed with androgenetic alopecia based on modified Norwood Hamilton classification Grade 2-5, aged 20-40 years were included in the study. The approval was taken from ethics committee.
Exclusion criteria was patients aged more than 50 years, unwilling for the procedure, Patients with any other type of alopecia, Patients with keloidal tendency, malignancies, platelet disorders, anaemia, bleeding disorders, HIV and HbsAg positive serology.
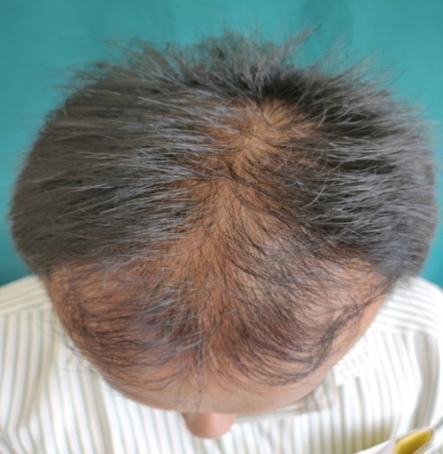
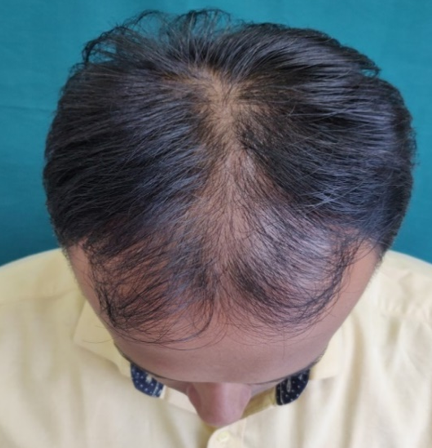
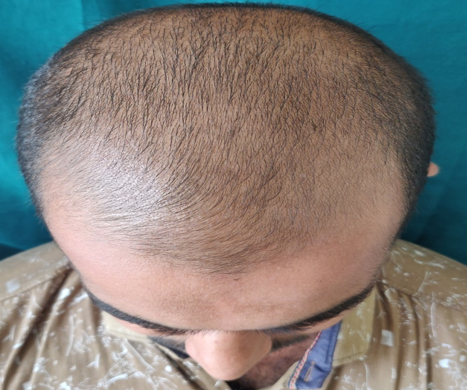
Complete history taking and detailed clinical examination was done in all the patients. Patients were explained everything about the study and procedure. Patients were put into two equal groups of thirty-one each. Patients in Group A were treated with 5% topical minoxidil and Group B patients were given 5% Minoxidil and platelet rich plasma therapy. All the necessary laboratory investigations were performed prior to the study. PRP was prepared using an automated centrifuge machine. About 18 ml of fresh blood is collected from median cubital vein and distributed equally in 2 conical test tubes each containing 1 ml of sodium citrate solution. Initially the tubes are subjected to “light spin” i.e., 1500 rpm for 18-20 minutes. This separates the blood into three layers, lowermost RBC layer, intermediate buffy coat containing WBCS and uppermost straw coloured platelet poor plasma (PPP).The buffy coat along with PPP is again subjected to “heavy spin” 3000 rpm for 10-12 minutes. This allows the platelets to settle at the bottom as a pellet. The upper two-third layer containing PPP is discarded and the lower one third is retained. The platelet pellet is re-suspended to form platelet-rich-plasma (PRP). Then PRP will be loaded in 1ml insulin syringes and injected over affected area by Nappage technique4:- Inter-follicular injections at the amount of 0.05 - 0.1 ml/cm2 in a retrograde fashion from deep to superficial, in a linear pattern 1 cm apart under proper aseptic precaution. A total of 4-10 ml of PRP wilI be injected. A total of 6 sessions at 4 week interval will be done on all the patients. 5% Minoxidil 1ml BD application daily will be advised for all patients after each PRP. Then 1 month follow up will be done after last sitting.
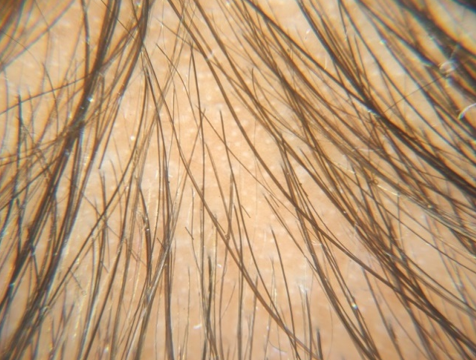
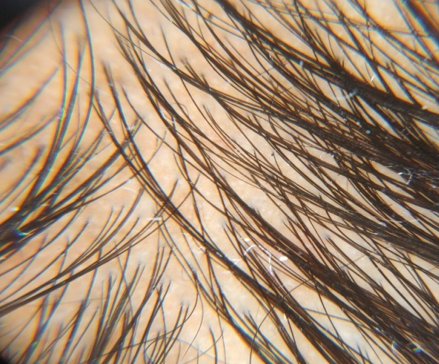
Assesment was done based on hair pull test, global standardised photos on every visit, subjective assessment with help of patient satisfaction questionnaires.
Results
Total of 62 patients participated in the study. The age of the study participants was between 20-40 years, mean age of the patients in Group A was 26.90 years and in Group B was 28.55 years with a standard deviation of 4.49 and 5.20 in Group A and Group B respectively. In Group A 51.6% and in Group B 58.1% had family History of Androgenic Alopecia.
Duration of AGA in majority of patients 25.8% in Group A and 35.5% was for more than 1 year.
Table 1
Comparison of Duration of Baldness and Areas affected between Group A and Group B using Chi Square Test
45.2% of patients of Group A and 41.9 % of Group B was in Grade 3 of AGA as per Hamilton Norwood scale
Highly significant increase in hair density was achieved after 4 months of treatment. At T4 (Fourth Session of treatment) Group B showed higher hair density (42.97± 8.96) as compared to Group A (36.94 ± 11.57) which was statistically significant at P = 0.03.
Table 2
Comparison of mean Hair Density between 2 groups at different time intervals using Independent Student t Test
At T1 mean Hair Pull Test score for Group A was (8.58 ±3.80) and for Group B was (8.65± 3.47) however the mean difference between hair pull test for these two study groups was not statistically significant. But from T2 sitting Group B showed significantly lesser hair pull test score (8.10 ±3.19) as compared to Group A (10.65± 4.61) which was statistically significant at P=0.01. From T2 until T7 Group B showed statistically significant lesser Hair Pull Test as compared to Group A
Table 3
Comparison of mean Hair Pull Test scores between 2 groups at different time intervals using Independent Student t Test
Most common adverse effect encountered during the study was Pain due to injection of Prp and few patients had complaints of headache after initiating topical minoxidil therapy, this was found to be 4(12.9%) in Group A and 25(80.6%) in Group B which was statistically significant with p value <0.001. About 9.7% of patients in Group A and 25.8% of patients in Group B experienced scalp pruritus, hypertrichosis was complained by 19.4% of patients in group A and 9.7% of patients in Group B.Contact Dermatitis was noted in 3.2% of the patients in both Groups ,Other adverse effects encountered by the patient includes erythema, active infections or hair fall post procedure.
Table 4
Comparison of Adverse effects of the Treatment among subjects between Group A and Group B using Chi Square Test
Note: Others include Erythema, Hyperpigmentation,active scalp infections.
1n Group A 25.8% was satisfied and 6.5 % was highly satisfied with the procedure and in Group B 35.5% was satisfied and 29.0% was highly satisfied with the procedure which was statistically significant P at 0.005.
Table 5
Comparison of Level of Satisfaction of treatment between group A and group B using chi square test
Discussion
Alopecia is a term used to determine loss of hair which results in diminution of hair 11 over the vertex and frontal recession or patterned hair loss. 12 Male baldness also known as Androgenetic alopecia (AGA) is a very common condition which results in severe hair loss. 13 It is a genetically determined non-scarring type of alopecia which is progressive in nature and is due to miniaturization of hair follicles. 14 It is a hereditary, multifactorial disorder mostly associated with low levels of androgenic hormone dihydroepiandrosterone (DHEAS). This condition is associated with transformation of terminal hair into vellus hair gradually which leads to shortening of hair follicles and affects the phases of hair cycle.
Although many treatments are available Topical minoxidil and oral finasteride are FDA approved and requires use for a longer duration of time and are associated with various side effects like hypertrichosis but both of them doesn’t restore hair completely.15 Minoxidil was developed originally for treatment of hypertension and is a vasodilator, acts over the potassium channels 16 which acts over the arterioles through various mechanisms to reduce the peripheral resistance.17 It helps to lengthen the anagen phase, increases the size of hair follicle and shortens the period of hair cycle.18 Oral minoxidil was associated with certain side effects like weight gain due to retention of water but on further trials and investigation 5% topical solution showed no side effects and was better than oral minoxidil. 16
Platelet rich plasma (PRP) is being increasingly used in AGA.PRP is a product formed by the processing of autologous blood to produce concentration of platelets. This product is derived from the platelets after its activation which in turn releases alpha granules and is rich in multiple growth factor 19, 20, 21 like platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF) ,epidermal growth factor (EGF) and interleukin (IL)-1. These growth factors help in inducing the proliferation of cells of dermal papilla. Anti-apoptotic effects of activated PRP has also been proposed as one of the factors stimulating hair growth. 22, 23, 24 PRP increases proliferation of dermal papillae cells through stimulation of protein kinase B and extracellular regulated kinase (ERK) signaling pathways, which contribute to apoptosis and cell growth regulation. 25
This is a Prospective Randomized Control Study in which a total of 62 patients are selected and divided into 2 Groups. Group A treated with Topical 5% Minoxidil only and Group B with Topical 5% Minoxidil along with monthly sessions of PRP treatment. In this study all the patients were between 20 to 40 years of age. Overall mean age was 27.75 years. Mean age in study by Shruti Gupta et al 26 (2017) was 28.3 years which is comparable with our study. Concerning family history of AGA, 51.6% of patients in Group A and 58.1% of patients in Group B had positive history. This is in comparison to study by Neena Sawant et al 27 in 2009 which showed 65% incidence of family history in patients with AGA. This confirms the well-known fact that AGA is genetic and inherited from family. So it is wise to start the treatment early in patients with a family history of AGA. On comparison of Duration of Baldness between 2 study groups it was found that In Group A 32.3% had duration of AGA for less than 6 months, followed by 25.8% of patients who had duration for more than an year.
In Group B 35.5% of patients was affected with AGA for more than a year and 22.6% had AGA for more than 2 years. This was in comparison with the study done by Satyendra et al where the duration of male type baldness in majority of patients was from 1.1 to 5 years. 28
Hair Density (hair/dermatoscopic field) significantly increased in Group A from T4 (fourth session of treatment) (36.94±11.57), at T5 (39.26±11.70), at T6(40.81±11.94) and at T7 (42.55±12.50). Hair density showed a similar improvement in Group B also from T4 (42.97±8.96), at T5 (46.42±8.57), at T6(49.97±7.82) and at T7 (53.13±8.10) (P < 0.001) compared to the onset of therapy. Highest hair density recorded at both Groups was after 6 sessions, this was in comparison to the study done by Maria-Angeliki Gkini et al where the hair density statistically increased from T3 to T6 compared to baseline T1.29
In our Study At T1 mean Hair Pull Test score for Group A was (8.58±3.80) and for Group B was (8.65± 3.47) however the mean difference between hair pull test for these two study groups was not statistically significant. But from T2 sitting Group B showed significantly lesser hair pull test score (8.10±3.19) as compared to Group A (10.65± 4.61) which was statistically significant at P=0.01. From T2 until T7 Group B showed statistically significant lesser Hair Pull Test as compared to Group A. This is in comparison with the study done by Prasanth et al where at the end of 6 months number of hair pulled became normal in group 1(Minoxidil with PRP) but in group 2(only PRP) few patients still showed breakage of hairs. 30
In studies which used manual double spin method, the yield of platelet varied from 2 ml to 12 ml. In our study, the final PRP was 6-7 ml. This is in contrast to Studies by Betsi et al 31 where automated PRP kits, which required lesser man power and facilitated the preparation of ready to apply platelet-rich suspensions in a reproducible manner was used.. However manual double spin method is cost-effective and platelet concentrations obtained matched the standardised values.
Regarding the use of activator in PRP, we didn’t use any activator in contrast to studies done by kuldeep et al were calcium gluconate was used, similarly in studies by Gkini et al.29 same activator was used. While other studies such as Khatu et al. 32 and Ubel et al 33 used calcium chloride as an activator. Some studies such as Betsi et al. 31 and 'Greco and Brandt' et al 34 have not mentioned the use of activator in their study.
On comparison of Adverse effects between two groups Pain was the most common side-effect noticed by the patients during the procedure. As scalp is highly innervated and PRP involved multiple injections, about 25patients (80.6%) in Group B experienced pain and about 4 patients in Group A complained of Headache after topical application of 5% minoxidil. Pain could be decreased by applying thick layer of topical anaesthesia for longer duration of about 90 minutes. Also nerve blocks of supraorbital, supratrochlear, temporal and posterior auricular nerves can be done to completely anaesthetize the areas of scalp. However, most of the patients tolerated pain with topical anaesthesia alone. Some patients complained of mild bleeding which subsided within few minutes and they were assured that some bleeding is necessary for the platelets to get activated. Other adverse effects include scalp pruritus, hypertrichosis contact dermatitis none of which was statistically significant. This is in comparison to the study done but satyendra et al 28 where the most common side effect encountered was pain followed by scalp pruritus, hyperpigmentation and increase in facial Hair.
In our study 51.6% in group A and 77.4% of patients from group B would like to recommend the procedure to others.
We used satisfaction questionnaire to assess the grade of satisfaction. Satisfaction was graded as not satisfied, just satisfied, satisfied, very satisfied and extremely satisfied About 25.8% in Group A and 35.5% in Group B was Satisfied, and about 6.5% in group A and 29.0% in Group B was highly satisfied with the procedure. 13(41.9%) patients in Group A and 2 (6.5%) in group B were not satisfied with the treatment. 8(25.8%) patients in Group A and 11(35.5%) in Group B were just satisfied with the treatment. None of the patients were extremely satisfied This is in comparison with the study done by Satyendra et al 28 where Improvement in patient’s satisfaction was found to be maximum in group II followed by group IV and group I, whereas none of the patients were satisfied in group III(Group I: Topical minoxidil 5% only; Group II: PRP with topical minoxidil 5%; Group III: NS only; Group IV: PRP only)
Limitation
Long term follow up of the cases was not done. Hair counts were not done over different parts of the scalp, nor were measurement of ratios of terminal to vellus hairs was done. The effects of treatment on anagen and telogen hairs were not observed .
Conclusion
The psychological and cosmetic importance of hair is immense in our society. Disruption in normal appearance of hair can predispose to low self esteem and negative body image. AGA is the most common cause of hair loss.
In our study we concluded that, both Minoxidil and Platelet rich plasma injection for androgenic alopecia is a simple, cost-effective and feasible treatment option for hair loss and can be regarded as a valuable treatment modality for androgenic alopecia. The results of the present study demonstrated that it definitely reduces hair fall and causes increase in thickness of existing hairs. Considering its excellent safety profile and relatively low cost, PRP along with minoxidil is a promising treatment option for patients with thinning hair. It works well in patients with lower grade of alopecia (grade III) but not in patients with severe grades.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms.In the form the patients have given their consent for their images and other clinical information to be reported in the journal.The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, lpbut anonymity cannot be guaranteed.