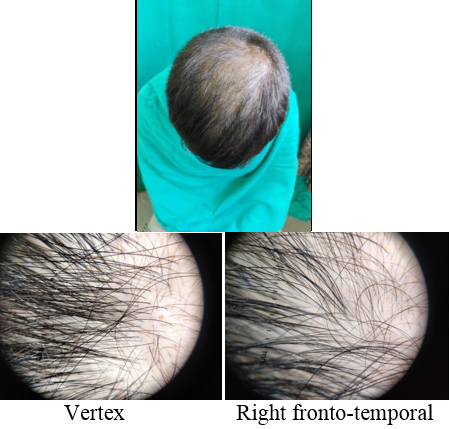
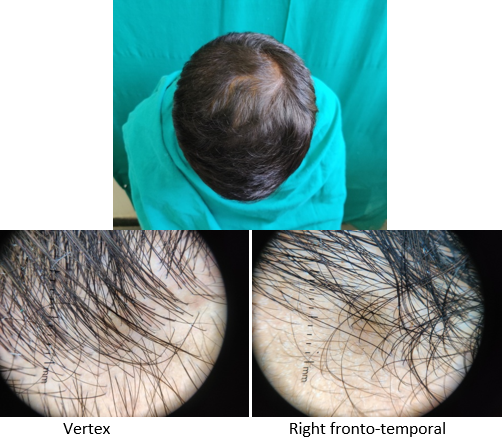
Introduction
Androgenic alopecia, commonly referred to as male-pattern hair loss in men or as female-pattern hair loss in women, affects at least half of all men by the age of 50 years, and more later in life.1 It is a distressing dermatological disorder characterized by hair loss. Although hair serves no critical physiological function in humans, hair loss can be psychologically devastating and adversely affect the self confidence of the patient.2
Minoxidil and finasteride 1 mg are the only drug specifically approved by Food and Drug Administration (FDA) for treating AGA either alone or in combination.3, 4 Efficacy of minoxidil in the management of male and female AGA is well established but long term application is usually required as the increased hair growth is first noticed at 4-6 months of treatment and the therapy has to be continued to maintain the efficacy.5 Main drawback of therapy is that the drug is slow to act leading to poor compliance and reversal of AGA. Whereas, local side effects like irritation, scaling, dryness and contact dermatitis limits its use.5
Platelet rich plasma (PRP), is emerging therapy for AGA. Platelet rich plasma (PRP) therapy used to treat AGA in last few years has shown promising results. PRP is autologous preparation of platelets in concentrated plasma.6 It contains more than 20 growth factors of which most important growth factors include PDGF, TGFβ, VEGF, IGF 1 along with their isoforms.6 Apart from various growth factors, PRP contains plasma proteins fibrin, fibronectin and vitronectin.7 In AGA, PRP induces differentiation of stem cells, prolong survival of dermal papilla cells, prolong anagen phase of hair cycle, and increases perifollicular vascular plexus by multiple mechanism.8, 9 Unlike conventional minoxidil therapy which has to be applied twice daily, PRP is given intradermally at an interval of 4 weeks which is convenient to the patient without significant adverse effects.
This study was aimed at comparing the clinical efficacy of PRP therapy and minoxidil fortified with finasteride therapy which may help us to know that which is more efficacious, acceptable, and safe form of therapy in the management of AGA.
Materials and Methods
This prospective interventional study was conducted in the Department of Dermatology, Venereology & Leprosy, Dr R. P. Govt. Medical College, Kangra (Tanda), H.P. after approval from institutional ethical committee for a period of one i.e. from 1st May 2019 to 31st May 2020. In first six months patients were recruited and followed up for next six months. The last patient was recruited 6 months from the day of start of study.
Exclusion criteria
Dermatological disease of the scalp
Patients with unrealistic expectations from treatment
Patients with a tendency for keloid formation
Patients with history of past treatment with oral finasteride or PRP
Patient with any hematological/coagulation disorders or on anticoagulation therapy
Patients who are immunosuppressed (HIV, malignancy, steroid or chemotherapy)
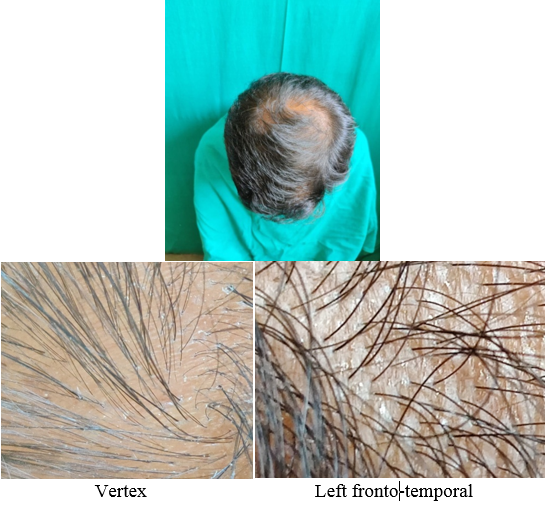
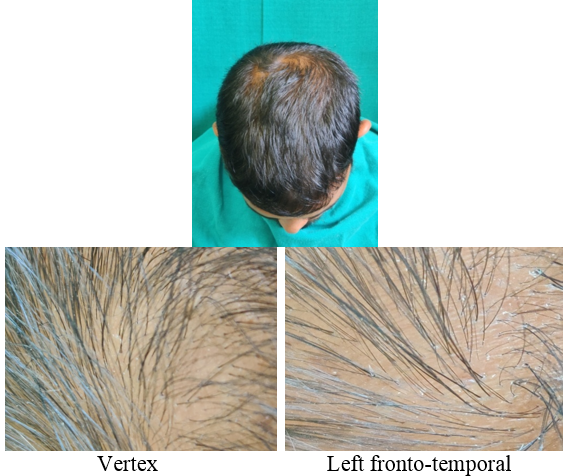
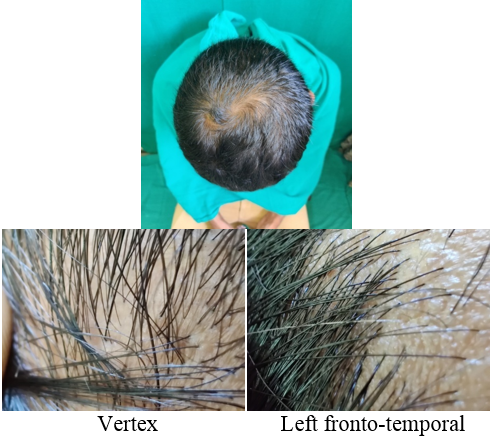
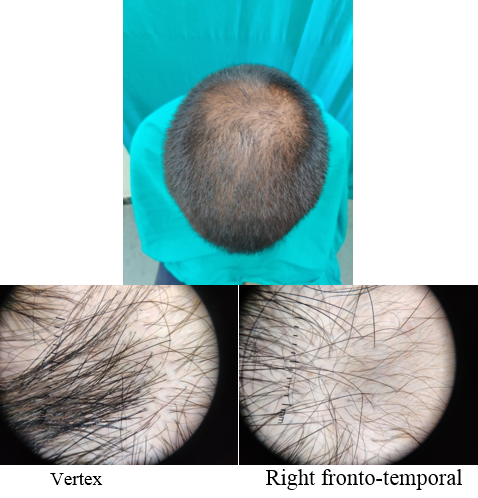
A detailed history was taken to rule out other causes of hair loss such as telogen effluvium, history of any drugs, or history of any systemic disease. The patients were asked about lifestyle‑related factors such as smoking and ultraviolet exposure which can aggravate AGA. Patients were asked for family history of AGA. A thorough clinical examination was performed in eligible patients to rule out any local dermatological disorder of the scalp, and a diagnosis of AGA was made; grade of AGA was assigned as per the Modified Norwood–Hamilton criteria.
Enrolled patients were divided into two groups – Groups A and B. Group A underwent monthly injections of PRP for once in 1 month for 4 cycles (0,1,2,3 months) followed by booster doses at 6 months and 12 months while Group B patients were treated with topical minoxidil (5%) fortified with finasteride (0.1%) therapy 1 ml applied twice daily for 12 months.
PRP preparation
After obtaining informed consent 8.5 ml blood sample was aspirated using 18G needle and collected in ACD vaccutainer tube. The first centrifugation or “soft spin” was carried out at 1200 rpm for 5minutes, and the separated buffy coat with PPP was collected with the help of a pipette in another test tube. This tube underwent a second centrifugation, a faster “hard spin at 2400 rpm for 15 minutes. The upper layer containing PPP was discarded and the lower layer of PRP was taken for platelet count.
Administration of Various Therapies
One hour prior to the administration of PRP, local anaesthetic cream was applied over the area of the scalp to be treated and cleaned with spirit and betadine.
With the help of an insulin syringe PRP was injected by intradermal technique (multiple small injections in a linear pattern 1mm apart) under proper aseptic precaution in minor operation theatre in the androgen-related areas of scalp.
Patients allocated to the minoxidil group were told to apply 5% minoxidil fortified with 0.1% finasteride 1ml twice a day and followed up similarly.
At each visit, hair count was checked over the prefixed square area. The patients were further evaluated at the end of 12 sittings.
Observations
In the present study comparing topical application of 5% minoxidil fortified with finasteride (0.1%) lotion with intralesional injection of PR, 60 cases of male pattern alopecia, who attended the Department of Dermatology between 1st May 2019 to 31st May 2020 were included. 30 cases of AGA each were included in both groups.(Table 1)
Table 1
There were a total of 60 patients with 30 patients in each group. The mean±SD age of patients in group A was 27.73±5.94 years and in group B was 24.47±4.0 years. Mean±SD age of onset of alopecia was 23.3±3.32 and 21.96±2.95 years in group A and group B respectively. The groups were comparable in terms of age of onset of AGA with p value of 0.103. There were 13 cases of AGA in PRP group who had history of alopecia in family while in group B there were 7 patients with similar history. In group A, there were 2 patients who were both smoker and alcoholic whereas in group B, there were 3 smokers and 8 patients who were alcoholic and out of them 1 patient was both smoker and alcoholic. Clinically, and according to Hamilton–Norwood Classification, the most frequent pattern of baldness in our study was grade 2 which was present in a total of 34 subjects with 13 subjects in group A and 21 subjects in group B followed by grade 3 present in 8 subjects in group A and 5 subjects in group B. There was no significant relation of grade of AGA with the study group
Patient scale
Mean±SD patient satisfaction score at baseline was 4.17±1.05 and 4.07±1.14 in group A and group B respectively and both the groups were comparable (p value 0.726). At end of 12th month the mean score increased to 5.23±1.04 in group A while in group B it increased to 4.12±1.35. There was significant difference in patient satisfaction in PRP group after treatment completion but no difference was observed between the two groups.(Table 2)
Investigator scale
On investigator satisfaction scale from photographs and dermoscopic microphotographs, the mean±SD score was 3.87±0.93 in group A while in group B, the mean score was 3.77±1.07. The groups were comparable in terms of investigator scale (P value 0.7). After 12th month, the mean±SD score in group A increased to 5.2±1.12 while in group B the score increased to 3.9±1.34. There was significant difference on investigator satisfaction scale score in PRP Group before and after treatment and between the groups after treatment (P value <0.001).(Table 3)
Final interpretation
After treatment for 12 months with the allotted treatment, hair growth was analysed and it was observed that 1 patient in group B showed no improvement while none was there in group A with any improvement. 22 patients in group A and 21 patients in group B showed 0-25% increase in hair growth. 26-50% improvement was observed in 8 patients in each of the group. The difference in hair growth improvement was not statistically significant.(Table 4)
Discussion
AGA is an androgen-dependent condition that affects men more than women. It has negative impact at the psychosocial level. Patients diagnosed with AGA can have significant impairment of quality of life, since hair is an important feature of self-image. It may even lead to depression and other negative effects. 10
There have been many studies done in past to demonstrate the efficacy and safety of minoxidil fortified with finasteride and PRP therapy independently however, there has not been much in the literature about the comparison of both forms of therapies. Hence, in this study, we compared minoxidil fortified with finasteride therapy with relatively newer PRP therapy so as to conclude which is more safer, convenient and efficient form of therapy in the treatment of AGA.
PRP can be prepared by manual double‑spin technique as well as with the help of automated devices. PRP was prepared by manual double‑spin method in the present study, and similar methods of PRP preparation were also adopted by Verma K, 11 Vats G 12 and Singh SK. 13
Even though PRP therapy has been used for over a decade in AGA, there are still no standard treatment protocols regarding number of sessions and interval between the two sessions for giving PRP therapy. We gave PRP therapy at a monthly interval for 4 months, which is, 4 sessions 1 month apart followed by follow up sessions at 6th and 12th month.
In our study, patient satisfaction score at end of 1st month i.e. baseline was 4.17±1.05 in group A which increased to 5.23±1.04 at end of 12th month as visualised by patient while in minoxidil group the values increased from 4.07±1.14 at baseline to 4.124±0.35 at 12 months with no significant change (p value=0.877) whereas on investigator assessment scale at the end of one month, baseline was 3.87±0.93 in group A which increased to 5.2±1.12 at the end of 12 months with significant p value=0.001.while in group B the value increased from 3.77±1.07 to 3.9±1.34 with no significant change(p value=0.679). At the end of 12 months, there was significant increase in group A in comparison to group B with p value=< 0.001).
In similar study, by Verma K,11 patients in group A (PRP), the mean patient satisfaction score 6.56±1.09 was significantly higher (p value=<0.001) than patients in group B (minoxidil) which was 4.85±1.46.
In another study by Singh SK,13 maximum improvement in patient self assessment at the end of the study found in PRP with minoxidil group followed by the PRP alone group and minoxidil alone group(p value<0.001).
In study done by Rai PB,14 topical minoxidil and fenestride was found to show significant improvement in quality of life.
A study conducted by Tanglertsampan15 comparing the efficacy of 3% minoxidil alone and combination of 3% minoxidil+0.1% fenastride topically in treatment of AGA revealed combination to be better than minoxidil alone.
PRP alone is “biology‟ and therefore the results depend on patient’s state of health and age. The older the patient’s cells, the ability to react and regenerate is worse, so not only the extent and severity of alopecia but also the age of patient should be taken into account.
To the best of our knowledge, this was the first prospective study comparing the efficacy of PRP and minoxidil fortified with finasteride therapy for the treatment of AGA where we concluded that intradermal PRP therapy (Group A) was better than topical minoxidil and finasteride therapy (Group B). PRP patients had a better outcome on patient satisfaction score and investigator assessment scale. Side effects in patients in PRP group were mainly pain at injection site. No significant side effects were observed in Group B patients except for mild scaling.
Conclusion
PRP is an exciting new non surgical therapeutic option for hair growth and stimulation in patients of androgenic alopecia. PRP therapy can be a valuable adjuvant to topical minoxidil therapy in the treatment of AGA. Side effects with PRP therapy were minimal with better results which may improve the compliance of the patient. It can be especially preferred in those patients who are unsatisfied or are noncompliant to regular use of minoxidil and finasteride therapy. Thus, both forms of therapies can be combined together for improved compliance and better outcome.
As PRP is in early stages of scientific research in hair restoration, it is a promising non surgical therapeutic option for those patients with androgenic alopecia. As of this date, there have been no large clinical studies to prove that PRP can positively affect hair growth on the long term. Overall, PRP seems to be a promising therapeutic modality but the level of evidence as of now, from the available published data is low. This demands further studies to gain more evidence before it is used more extensively.
Limitations
Major limitation of this study was a small sample size, hair counts were not done over different parts of the scalp, nor were measurement of hair thickness and vellus hair counts. The effects of treatment on anagen and telogen hair were not observed.