Introduction
Platelet-rich plasma (PRP) is an autologous preparation of platelets in concentrated plasma (with usually>1,000,000 platelets/μL or 2–7 times the concentration in whole blood).1 PRP contains various growth factors in the alpha granules of platelets, such as platelet-derived growth factor, vascular endothelial growth factor, epithelial growth factor, fibroblast growth factor, hepatic growth factor, transforming growth factor, etc. These growth factors play a role in tissue repair, regeneration, angiogenesis, connective tissue remodelling and cell cycle regulation.2
The method known as differential centrifugation is used for PRP preparation. Apart from dermatology, PRP is used in various medical specialities like orthopaedics, maxillofacial surgery, regenerative medicine, gynaecology, dentistry and sports medicine.3, 4 In dermatology, PRP is used in alopecia (angiogenic role), skin rejuvenation (increases dermal elasticity), acute and chronic ulcers (enhanced re-epithelisation by growth factors), acne scars and post-surgical scars(collagen synthesis), lipodermatosclerosis, etc.5
Although PRP has been used for many years, no standard preparation protocol exists. Therefore, attaining a count of>1,000,000platelets/μL without using expensive PRP kits in a clinic or even a tertiary care setting remains a hurdle. Hence, this study aims to analyze the platelet count of PRP prepared using various centrifugation parameters to achieve the ideal PRP.
Materials and Methods
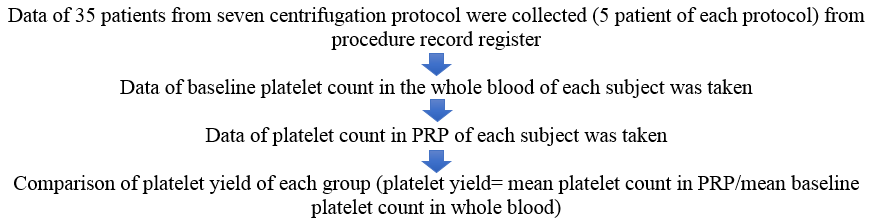
We conducted this cross sectional study at the dermatology outpatient department of a tertiary care hospital from data of the period of 10 months from March 2022 to December 2022. The study was approved by the Institutional Ethics Committee of the Hospital. A data of 35 apparently healthy individuals who were enrolled for PRP therapy for various dermatological indications like androgenic alopecia, acne scars, melasma, etc. was collected.
Inclusion criteria
Patients of all age groups and both genders enrolled for PRP therapy for various dermatological indications e.g. androgenic alopecia, acne scar and melasma.
Statistical analysis
The data was analyzed using statistical software - SPSS version 26 (IBM Corp. released 2019. IBM SPSS Statistics for Windows, Version 26.0 Armonk, NY: IBM Corp.). The platelet yields derived from different centrifugation speeds were compared using non-parametric tests (Kolmogorov Smirnov test & Shapiro Wilk test).
Charts and tables were prepared using Microsoft Word® 2010, Microsoft Office Professional Plus, and Microsoft Corp.
Sample size: Total- 35
Table 1
Protocols of PRP preparation
Procedure of PRP preparation
The whole procedure of PRP preparation was carried out in an air-conditioned environment at 24 degrees Celsius.
The rotor's size and radius (R) vary with different centrifuge machines. Therefore, relative centrifugal force (g) values are converted to rotation per minute (rpm). The conversion factor from ‘g’ to rpm is as follows4:
g = (1.118 x 10-5) R (rpm)2
Peripheral blood (20 cc) was obtained from each patient, out of which 3ml was added to an EDTA (Ethylenediaminetetraacetic acid) tube for baseline platelet count, and the rest of it was divided into 2 BD (Becton Dickinson) vacutainer whole blood tube (Acid Citrate Dextrose-A, Yellow, 8.5 ml) for PRP preparation. PRP was prepared in a Remi Medico Plus centrifuge machine (Figure 2)(Radius of centrifuge-13 cm) using a two-step centrifugation method.
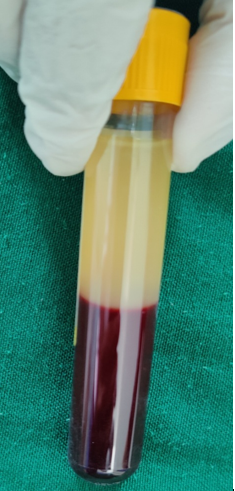
The first spin is performed according to the centrifugation speed and time allotted for that subject. After the first spin, the whole blood separates into three layers: an upper layer containing platelet-poor plasma (PPP), an intermediate layer called buffy coat rich in WBCs and platelets and a bottom layer containing RBCs as shown in Figure 4. The upper layer, along with the buffy coat, is transferred into a sterile plain vacutainer and placed in the centrifuge machine for the second spin.
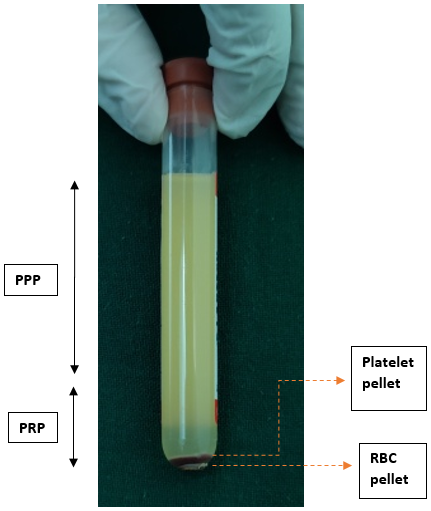
The second spin is performed with predetermined centrifugation speed and time. The resultant plasma contains platelet-poor plasma (PPP) in the upper 3/4th volume and platelet-rich plasma (PRP) in the lower 1/4th volume, as shown in Figure 5.
The upper 3/4th portion (PPP) is withdrawn with the syringe and discarded, while the lower 1/4th portion (PRP) is resuspended to form a homogenized PRP, as shown in Figure 6.
Figure 5
Upper 3/4th PPP is discarded, and lower 1/4th PRP containing platelet pellet and RBC at the bottom is resuspended to form homogenized PRP

The seven protocols selected were inspired by the previous studies with few modifications, which were conducted to determine optimum centrifugation speeds for PRP preparation.6, 7, 8, 9, 10, 11 The data from seven groups containing an equal number of samples (5 samples in each group) were collected in this study. Various centrifugation protocols used in our study are given in Table 1.
In each of the study groups, the mean platelet count of the whole blood and PRP are calculated, and the platelet yield is obtained.
Results
Table 2
Results obtained in various spin variations
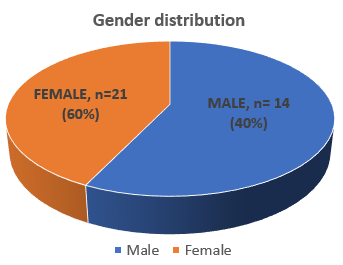
Table 2 depicts the baseline platelet count, PRP platelet count and platelet yield, as well as the averages for all seven spin variations used in our study. The data consisted of 35 samples from 35 individuals, comprising40% males (n=14) and 60% females (n=21), as shown in Figure 7. They were in the age group of 18–40 years. Androgenic alopecia was the most common indication, followed by acne scar.
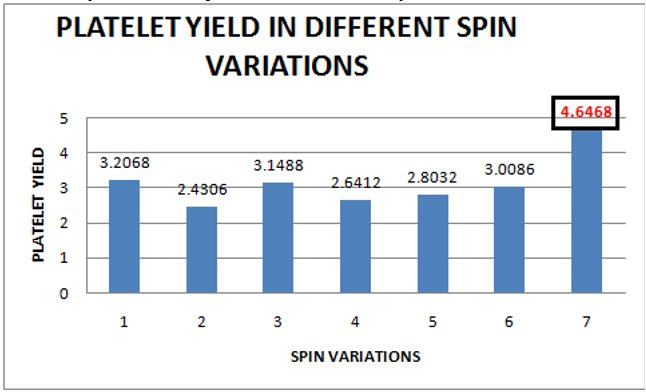
In our study, the highest mean platelet count was achieved by applying spin variation-7 (1300 rpm/246 g for 20 minutes followed by 1600 rpm/373 g for 20 minutes), which was 13,31,400/cumm. The second highest mean platelet count was achieved by spin variation - 1 (1020 rpm/151 g for 20 minutes followed by 1780 rpm/461 g for 10 minutes), which was 9,91,200/cumm as shown in Figure 8. Therefore, the highest platelet yield is achieved by spin variation-7, which is 4.6468, followed by spin variation- 1, which is3.2068, as shown in Figure 9.
Figure 7
Graph showing mean baseline platelet count versus mean platelet count in PRP in each study group

The platelet yields derived from different centrifugation speeds were compared with each other using non-parametric tests (Kolmogorov Smirnov test & Shapiro Wilk test). Since the sample size is small (< 40) so, data is checked for Shapiro Wilk significance. It is >0.05, so data follows a normal distribution, as shown in Table 3.
Table 3
Tests of normality of data
Table 4
Descriptive data for all seven different centrifugation speeds
One-way Analysis of Variance (ANOVA) was used for analysis as data followed a normal distribution. It is followed by Tukey’s Honestly Significant Difference (HSD) post hoc test for multiple individual comparisons.
The test statistic is F(6, 28) =9.219 (p = .000). Using an α of 0.05, we get a critical value of F0.05;(6,28) = 2.445. Since the test statistic is much larger than the critical value, we reject the null hypothesis and conclude that a statistically significant difference exists. There was a statistically significant difference between spin variation - 7 when compared to spin variations - 1 to 6 as determined by one-way ANOVA, as shown in Table 5.
Table 5
ANOVA test
|
ANOVA |
|||||
|
Platelet Yield |
|||||
|
|
Sum of Squares |
df |
Mean Square |
F |
Sig. |
|
Between Groups |
15.782 |
6 |
2.630 |
9.219 |
.000 |
|
Within Groups |
7.989 |
28 |
.285 |
|
|
|
Total |
23.772 |
34 |
|
|
|
A Tukey post hoc test revealed that the simultaneous pairwise comparisons between spin variations - 1 to 6 are not significant (p ≥ 0.05), whereas the comparison between spin variation - 7 with respect to other spin variations is significant (p < 0.05), as shown in Table 6. Thus, platelet yield obtained was statistically significantly higher with spin variation-7 after comparing spin variations - 1 to 6 with each other as well as with spin variation-7 (4.6468 ±.71619) (p = .000). There was no statistically significant difference found during multiple comparisons between the spin variations - 1 to 6.
Table 6
Tukey’s HSD post-hoc test
Discussion
PRP was first described in 1997 by Whitman et al. as a derivative of the fibrin glue. 12 The therapeutic efficacy of a PRP preparation is largely determined by its platelet concentration. Therefore, clinicians should be aware of the fact that different PRP preparation protocols can produce different platelet concentrations, which can translate into a difference in efficacy and clinical response. 13 As per the study conducted by Dashore S et al., the recommended parameters of centrifugation for PRP preparation are100–300 g for 5–10 minutes for the first spin and 400–700 g for 10–17 minutes for the second spin. 4 Various factors like the type of anti-coagulant, centrifugal speeds, the amount and the kind of growth factors existing in PRP, the number of platelets in the donor’s blood and PRP are essential for determining the procedure's efficacy and clinical outcome. Therefore, they must be carefully considered before applying to clinical practice. 10, 14, 15, 16 Haynesworth et al. demonstrated that the proliferation and differentiation of adult mesenchymal stem cells were directly related to platelet concentration. A concentration of approximately 4 to 5 times the baseline platelet count would be required to produce a sufficient cellular response. 17 Rughetti et al. found that there is a bell-curve relationship between the platelet count and its functional activity. Therefore, the optimal proliferation and differentiation of endothelial cells occurred at 1.25×106 platelets/ml, and optimal angiogenesis occurred at 1.5×106 platelets/ml. As the platelet count increased higher than 1.5× 106 platelets/ml, the proliferation of endothelial cells showed a decreasing trend.18
A classification of PRP preparation was proposed by Dohan Ehrenfest DM, Rasmusson L, Albrektsson T in 2009 as follows:19
Pure platelet-rich plasma (P-PRP) or leukocyte-poor platelet-rich plasma: It contains no WBC and low-density fibrin network after activation.
Leukocyte and platelet-rich plasma (L-PRP): It contains WBCs and a low-density fibrin network after activation. Most of the commercially available systems belong to this group.
Pure platelet-rich fibrin (P-PRF)/ leukocyte poor platelet-rich fibrin preparation: It contains no WBC and a high-density fibrin network. They exist only in strongly activated gel forms and cannot be injected like traditional fibrin glues.
Leukocyte and platelet-rich fibrin (L-PRF)/second-generation platelet-rich plasma: It contains WBC along with a high-density fibrin network.
The buffy coat inclusion methods give a higher platelet concentration in PRP, but the disadvantage of this method is higher contamination with RBCs and WBCs. 3 The role of WBCs in PRP is not clear. It is believed that WBCs in PRP provide protection from infections, increase the release of growth factors and help in angiogenesis. 19 Also, as mentioned by Oudelaar et al., the concentration of vascular endothelial growth factor is significantly higher in PRP produced by systems with higher concentrations of platelets and leucocytes than in P-PRP kits. 20 In PRP, RBCs may affect platelet function by altering pH and promoting inflammation. Also, RBCs can act as a source of reactive oxygen species. When used for skin rejuvenation, RBCs may also induce post-inflammatory hyperpigmentation due to hemosiderin deposition. 21, 22, 23 But RBC contamination is quite unavoidable in the PRP preparation, including the buffy coat layer, even if care is taken while separating the buffy coat from a layer from RBC volume. There is no consensus on whether or not the platelet must be previously activated before their application and which activator to use. In some studies, platelets are activated using thrombin or calcium, while in other studies; platelets are not activated. 24
In today’s era, numerous centrifugation methods are available for PRP preparation. However, there is a need for more literature to determine the best method of PRP preparation. Some authors recommend the single spin method wherein the whole blood is centrifuged only once. Kahn RA et al. showed that centrifugation of 3731 g for 478 ml of whole blood for a period of 4 minutes was optimal to obtain the highest platelet concentration in the sample 8. However, many studies state that a double spin method of PRP preparation yields a higher platelet count. A comparison of our research with various protocols for PRP preparation by different authors is mentioned in Table 7.
Table 7
Comparison of various protocols of PRP preparation
|
S.No. |
Study |
1st Spin |
2nd spin |
Platelet concentration in PRP (x106 / cumm) |
% of the increase in platelet counts (times) |
|
1 |
Araki et al 25 |
230 – 270 g x 10 minutes |
2330 g x 10 minutes |
1.89 |
7.4 |
|
2 |
Amable et al 7 |
300 g x 5 minutes |
700 g x 17 minutes |
0.14 – 0.19 |
5.4 – 7.3 |
|
3 |
Tamimi et al 26 |
160 g x 10 minutes |
400 g x 10 minutes |
0.632 |
3.52 |
|
4 |
Bausset et al 27 |
130 g x 15 minutes |
250 g x 15 minutes |
- |
3.96 |
|
5 |
Our study |
246 g x 20 minutes |
373 g x 20 minutes |
1.47 |
4.6468 |
In our study, we have used the data of protocols with slightly modified spin parameters used in previous studies and studied the data of seven novel spin parameters. We have used the data of buffy coat inclusion method in our study. We compared the platelet yield instead of the mean platelet count obtained by each group so that we could avoid the bias created by the difference in the baseline platelet count of participants of each group. Here, the highest mean platelet yield is achieved by applying spin variation-7 (1300 rpm/246 g for 20 minutes followed by 1600 rpm/373 g for 20 minutes), which was 13,31,400/cumm (4.6468 times as compared to mean platelet count in the whole blood).
In our study, spin variation-7 produced the mean platelet count of PRP of more than 1,000,000/cumm, which, as described earlier, is required for producing optimal results by PRP. The spin variations 1,2, 3, 4, 5 and 6 have produced a platelet count of less than 1,000,000/cumm, so they may not produce the desired clinical outcomes.
Araki et al. used varying accelerations ranging from 230-270g in the first spin and 2330g in the second spin. They achieved the mean platelet count of 1.89 * 10 6 /cumm and a platelet yield of 7.4 times as compared to the mean platelet count of PRP of 1.33*10 6/cumm and platelet yield of 4.6468 times in our study. 25 Here, the first spin speed was comparable to our study, but the second spin speed was very high compared to our study. Also, the time used for PRP preparation is less compared to our study. However, few authors believe that the longer time periods slightly increase the platelet recovery and decrease the WBC in the upper layer. Hence, time can be a control parameter when low levels of WBCs are required in the PRP sample. 24
In our study, a higher spin speed is applied for the second spin than the first spin, which is in accordance with the other studies compared here.
To our knowledge, we found that there were many methods for preparing PRP, but no comparative study similar to the one shown in the present report has been described previously. The limitation of our study was a limited sample size; hence, further studies with larger sample sizes are required.
Conclusion
The science behind the preparation of PRP is still evolving. There is no single "ideal" method for the preparation of PRP. Multiple factors like temperature, cell counts of whole blood, centrifugation method parameters, and centrifuge machine parameters play a significant role in the quality of PRP prepared. In our study, the highest platelet count in PRP is achieved by spin variation-7 (1300 rpm/246 g for 20 minutes followed by 1600 rpm/373 g for 20 minutes), which was 13,31,400/cumm (4.6468 times as compared to the mean platelet count in the whole blood). It is necessary that dermatologist take into account various facets of PRP preparation to ensure that the best therapeutic result is delivered to their patients.