Introduction
Pemphigus Vulgaris (PV) is a chronic, recurrent, vesiculobullous disorder characterized by intraepithelial vesicles and/or bullae in the skin and mucous membranes. The mucosal dominant type of PV occurs due to auto-immune IgG antibody against Desmoglein-3 whereas both Desmoglein 1 & 3 are targeted in the mucocutaneous type of PV. 80-90% of patients with pemphigus vulgaris develop oral lesions and in 60% of cases, oral lesions are the first sign.1 The oral ulcers are highly debilitating for the patient and difficult to treat for dermatologists as compared to skin lesions. It has been observed that oral lesions tend to persist even after the skin lesions have subsided and a prolonged course of oral/topical/intralesional corticosteroids have to be given adding considerable side effects. Topical corticosteroids cause many complications like secondary fungal and bacterial infections and these erosions also disturb the day-to-day lifestyle of patients.
Autologous platelet-rich plasma (PRP) therapy contains a platelet-rich concentrate with a large number of growth factors like platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), epithelial cell growth factor (ECGF), insulin-like growth factor (IGF), fibronectin and vascular endothelial growth factor (VEGF)2 and has been used in the treatment of various non-healing ulcers like trophic ulcers, diabetic ulcers, neuropathic ulcers. 3 PRP promotes re-epithelialization by regulating the biological function of epidermal stem cells (ESCs). It significantly promotes the angiogenesis of wounded tissue and promotes wound contraction and stabilizes the collagen arrangement.4 PRP also stimulates the production of collagen and extracellular matrix. The wound healing property of PRP can be used to treat recalcitrant oral ulcers of pemphigus as it accelerates the healing process, prevents the patients from side effects of steroids, and reduces the pain and discomfort of the patients.
POLIS- Pemphigus Oral Lesion Intensity Score, is a Novel Scoring System for Assessment of Severity of Oral Lesions in Pemphigus Vulgaris.5 It evaluates 16 parameters of the disease and grades them as None (0), Mild (1), Moderate (2), Severe (3), Very severe (4). This scoring system has been used in the study to evaluate the baseline severity and monitor the response to I/L PRP therapy for recalcitrant oral ulcers in patients of PV.
Table 1
POLIS (Pemphigus oral lesion intensity score) 5
Materials and Methods
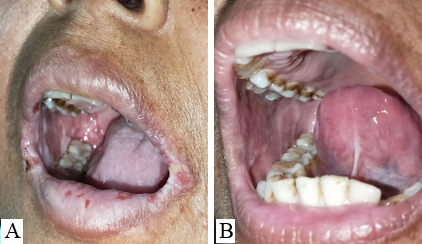
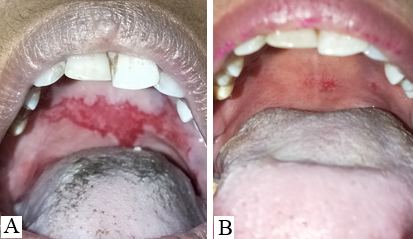
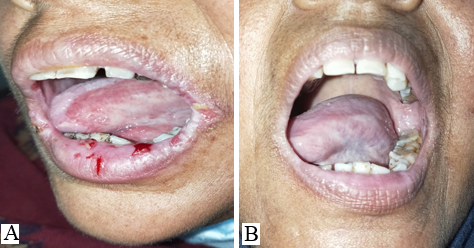
The study was conducted at a tertiary health care center in northern India for 2 years. All histopathologically proven cases of Pemphigus Vulgaris with recalcitrant oral erosions were included in the study. All the patients were treated with various modalities such as pulse dosage of corticosteroids, variable tapering doses of oral corticosteroids, injection Rituximab, immuno-suppressants like- Azathioprine, and MMF. In all such cases in which the skin lesions had almost healed (approx. 90%) but the oral lesions were still persistent and the patients were on partial remission at minimal therapy i.e. Tab Prednisolone 10 mg or equivalent and half dose of adjuvant therapy, 5, 6 were labeled as cases of Pemphigus Vulgaris with recalcitrant oral ulcers and included in the study. No topical treatment for oral ulcers was given during the study.
The baseline POLIS score was calculated and labeled as POLIS-0. All the cases included in the study were off all topical treatments for oral erosions. The cases were subjected to autologous intra-lesional PRP weekly for 3 sessions. For PRP preparation, the first centrifugation spin was done at 2400 rpm for 10 minutes and the second spin was done at 3600 rpm for 5 minutes. 5ml of freshly prepared PRP was injected intralesionally along the margins and base of the oral erosions using a tuberculin syringe. Then, the POLIS score was calculated at each session i.e. POLIS-1 at 7 days, POLIS-2 at 14 days, and POLIS-3 at 21 days. The result was evaluated by a change in POLIS score after each session. Results were analyzed using statistical software SPSS version 26.0. We performed a test of normality on our data set to guarantee the accuracy of our ensuing statistical analysis. The Shapiro-Wilk test was chosen due to the small sample (<50) of participants.
Results & Observation
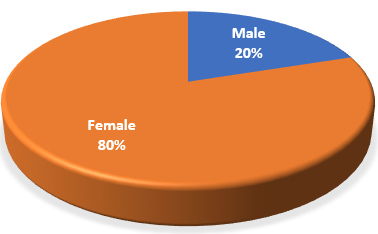
Out of the total 15 cases, 12 were female and 3 were male i.e. the gender ratio was 4:1 (Figure 1) (Table 2). The minimum and maximum age of the cases were 24 and 62 respectively (Table 2) with the mean age of participants being 40 ± 9.5 years.
Their disease duration ranged from 3 months to 2 years (Table 2).
11 patients had bilateral erosions in their buccal mucosa whereas 4 had unilateral erosions. Lips and gingiva were involved in 8 cases.
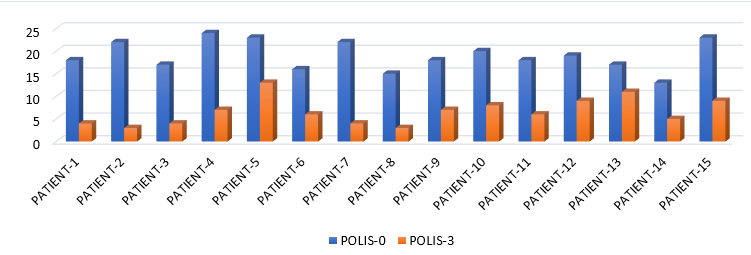
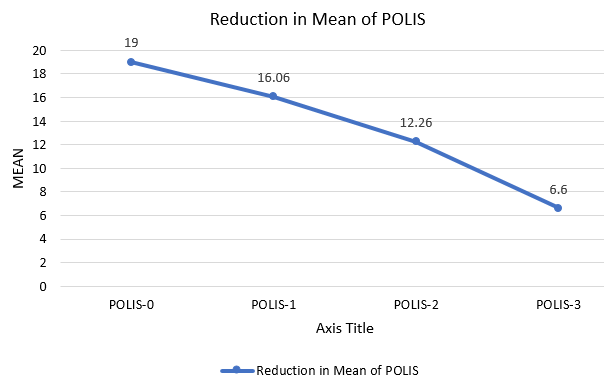
POLIS score ranges from 0 to 64.5 The mean of POLIS-0, POLIS-1, POLIS-2, and POLIS-3 for the oral lesions were 19, 16.06, 12.26, and 6.60, respectively. It was observed that there was a gradual reduction in POLIS score after subsequent PRP sessions (Figure 2) and pain during brushing, eating, swallowing, and talking reduced on the Likert scale after each PRP session. There was a significant difference between the mean POLIS-0 score and POLIS-3 score in the cases, from baseline and after 3 sessions of I/L PRP therapy, (p<0.001) (Figure 3).
No major side effects were seen in any patients. Pain, burning sensation, and swelling during injection were complained by a few patients that subsided after some time without any treatment.
The Shapiro-Wilk test yielded a p-value larger than 0.05, meaning that the normalcy null hypothesis could not be disproved.
These findings give our analysis and interpretations a strong basis. Our data's normal distribution improves the generalizability of our conclusions.
“The t-test was conducted to compare test scores between the POLIS-0 and the POLIS-3. There was a significant difference in test scores between the baseline POLIS-0 (M = 19.0, SD = 3.25) and the POLIS-3 (M = 6.60, SD = 2.97); t(13.33) = 14, p = <0.0001.” also showing the test is significant.
Table 2
Summary of the clinical characteristics of the PV patients
Discussion
The mucosal erosions of Pemphigus Vulgaris are often the most difficult sites to treat for dermatologists as compared to the cutaneous lesions.7, 8 The oral lesions severely hamper the day-to-day activity of the patients like brushing, eating, swallowing, and talking which affects the dietary intake of the patients. Improper nutrition of the patients delays the recovery and adds on to the disease burden. 9 Oral, I/L and topical corticosteroids have to be continued for a longer duration even after the skin lesions have subsided. The use of ILS has been found to be effective in treating recalcitrant oral erosions, 7 nonetheless, few patients do not respond to it satisfactorily. In this pilot study, we aimed to evaluate the efficacy of I/L autologous PRP in recalcitrant oral lesions of PV. It was observed that POLIS (Pemphigus oral lesion intensity score) significantly reduced after 3 sessions of I/L PRP therapy. Poor quality of life has a significant impact on treatment outcome of pemphigus. 10 Also, the overall quality of life of cases improved significantly.
PRP contains a milieu of growth factors & cytokines that promotes the healing process and has been used in treatment of chronic, non-healing ulcers being an easy, day care and cost effective procedure.11 PRP has also been used in various oral surgeries & regenerative endodontic procedures due to its effect of wound healing and maintaining pulp vitality.12
I/L Rituximab and I/L methotrexate have been tried for recalcitrant oral lesions with variable results. Vinay et al13 reported 3 OPV patients who had inadequate response to ILS but responded to I/L Rituximab injection. Another study conducted by Hrin, Mathhew et al on cases of mucous membrane pemphigoid showed promising results with I/L methotrexate.14
Taking into account that POLIS measures all the aspects of oral lesions of PV like sites, extent, duration, pain and difficulty in day to day activities; gradual reduction of POLIS in the study participants can be attributed to wound healing properties of PRP where it has shown to accelerate soft tissue healing. Also, it prevents the patients from side effects of I/L and topical corticosteroids like soft tissue atrophy, 15 secondary bacterial and fungal infections like-candidiasis, granuloma formation & gingival neovascularization.16
In conclusion, in this preliminary study, there appears to be significant improvement in recalcitrant ulcers of oral PV with I/L PRP therapy and no major side effects apart from pain and discomfort during injection was seen in the study participants. However, because of small sample size, further large-scale studies are needed to confirm our findings and explore the potential of PRP therapy as an adjunct to management of mucosal lesions of PV. We suggest that I/L autologous PRP must be used for treatment of mucosal erosions of PV cases who develop side effects to I/L and topical corticosteroids and when I/L and oral corticosteroids are contraindicated.