Introduction
Glomus tumor is a rare mesenchymal neoplasm accounting for less than two per cent of all soft tissue tumors. Ten percent of patients may show multiplicity and malignant glomus tumors are even rare.1
Glomus tumor stems from neuromyoarterial cells of normal glomus apparatus in the reticular dermis and is commonly a benign neoplasm.2
In 1812, Glomus tumors were first described by William Woods as ‘painful subcutaneous tubercles’. It was in 1924, that these lesions were named ‘Glomus tumors’ by Masson after studying their pathology.3 Glomus tumors arise from glomus cells of the glomus body which have contractile properties of smooth muscle cells. This glomus body is an arteriovenous anastomosis of a specialized form which regulates the circulation of skin and thus plays a role in thermoregulation.4, 5 Benign glomus tumors with diffuse forms of angiomatosis are termed glomangiomas.6
Digits, palms and soles of feet are locations where these glomus bodies are predominantly situated. This explains the predilection of glomus tumors for these sites.5, 7 The most common location of glomus tumors is subungual. 5, 8
Given the rarity of this tumor, there is a possibility of low suspicion and a missed clinical diagnosis, especially in extra digital locations. This may result in significant discomfort to the patient till an accurate diagnosis is made, as this lesion is extremely painful. Thus, it is essential to know the diagnostic clues and morphology of this lesion to increase the probability of timely diagnosis.
Materials and Methods
This was a retrospective study where the cases were retrieved from patient medical records and histopathology report records from the last nine years (January 2015 to January 2024). This case series included nine cases of histopathologically proven glomus tumors. Cases with incomplete records or ambiguous diagnoses were excluded. Data was collected, tabulated and analysed by descriptive statistics using Microsoft Excel 2016.
Results
There were a total of nine cases in this case series (Table 1 gives a summary of all our cases) of which five were men and four were women (Male to female ratio was 1.25:1). We observed a very wide age range of 19 years to 78 years (mean age 37.67± 17.5 years). The most common sites were digital parts of extremities (5/9, 55.56%). Four of these digital locations were subungual (4/5, 80%). Subungual glomus tumors were seen on the ring finger (2/4, 50%), distal phalanx of thumb (1/4, 25%) and index finger (1/4, 25%)]. Other sites were extra-digital sites [ankle (1/9, 11.11%), foot (2/9, 22.22% and suprapatellar region (1/9, 11.11%). The most common laterality was the left (5/9, 55.56%). Right laterality was seen in 4/9, 44.44%. All of our cases presented with painful swellings (9/9, 100%).
Table 1
Summary of all glomus tumor cases of this case series
Figure 1
Hematoxylin and Eosin (H&E) images of glomus tumor (Case 8) showing a well circumscribed tumor composed of uniform round to oval cells with monomorphic round nuclei and scanty eosinophilic cytoplasm arranged in nests. The stroma shows myxoid material. No nuclear atypia, necrosis or mitosis. (A -40X magnification, B-100X magnification, C- 400X magnification)
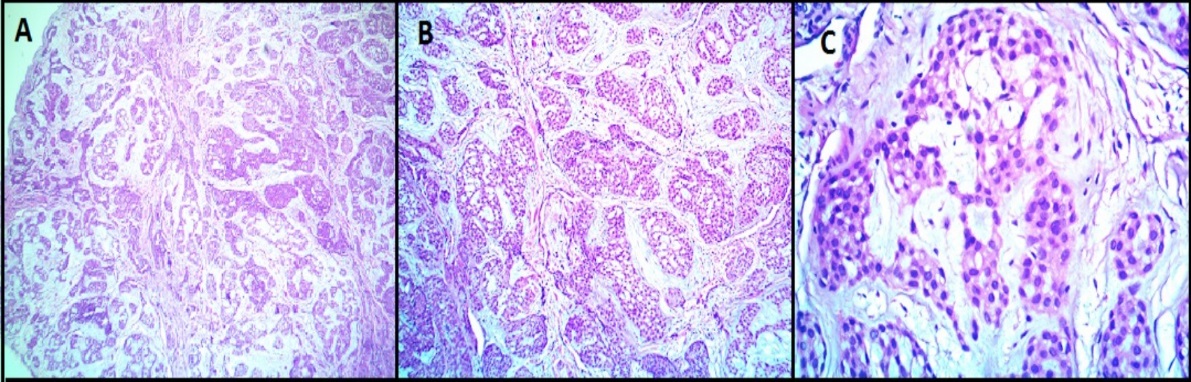
Figure 2
Immunohistochemistry (IHC) images of glomus tumor (Case 8) showing tumor cells expressing; A): Vimentin and C): Smooth Muscle Actin (SMA). Tumor cells are negative for B): Desmin (A,B,C- 100X magnification)
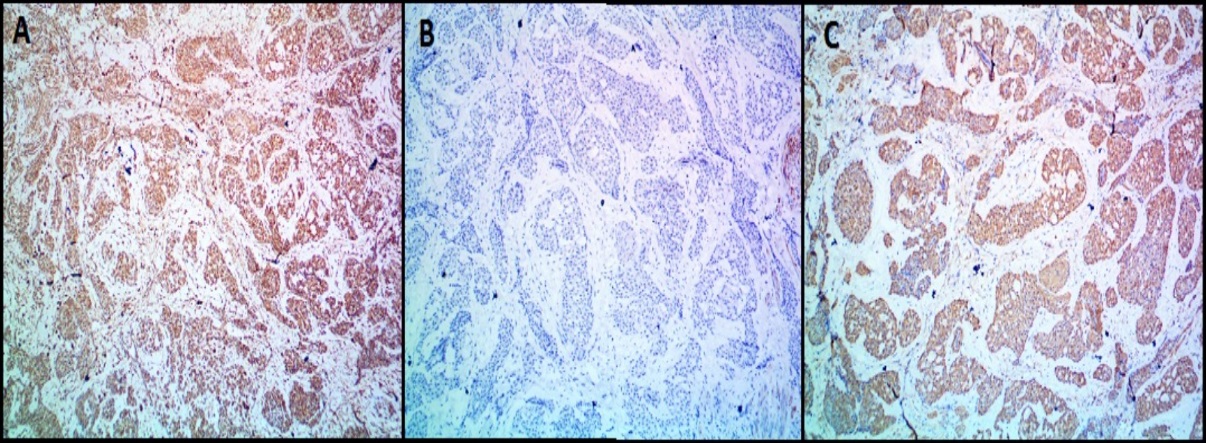
Figure 3
IHC images of glomus tumor (Case 8) showing expression of A): Calponin and B): High-molecular-weight Caldesmon (H- Caldesmon). (A & B-100X magnification)
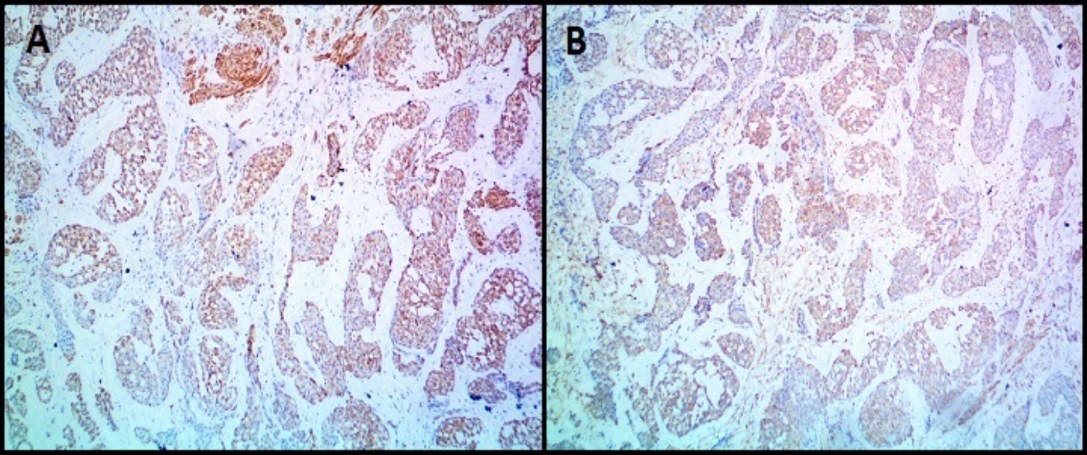
Figure 4
H&E images in a case of glomangiomatosis (Case 4) showing diffuse infiltrating multifocal tumor with prominent vasculature surrounded by glomus cells without nuclear atypia (A, B, C- 40 X magnification), D, E- 100X magnification, F-400X magnification)
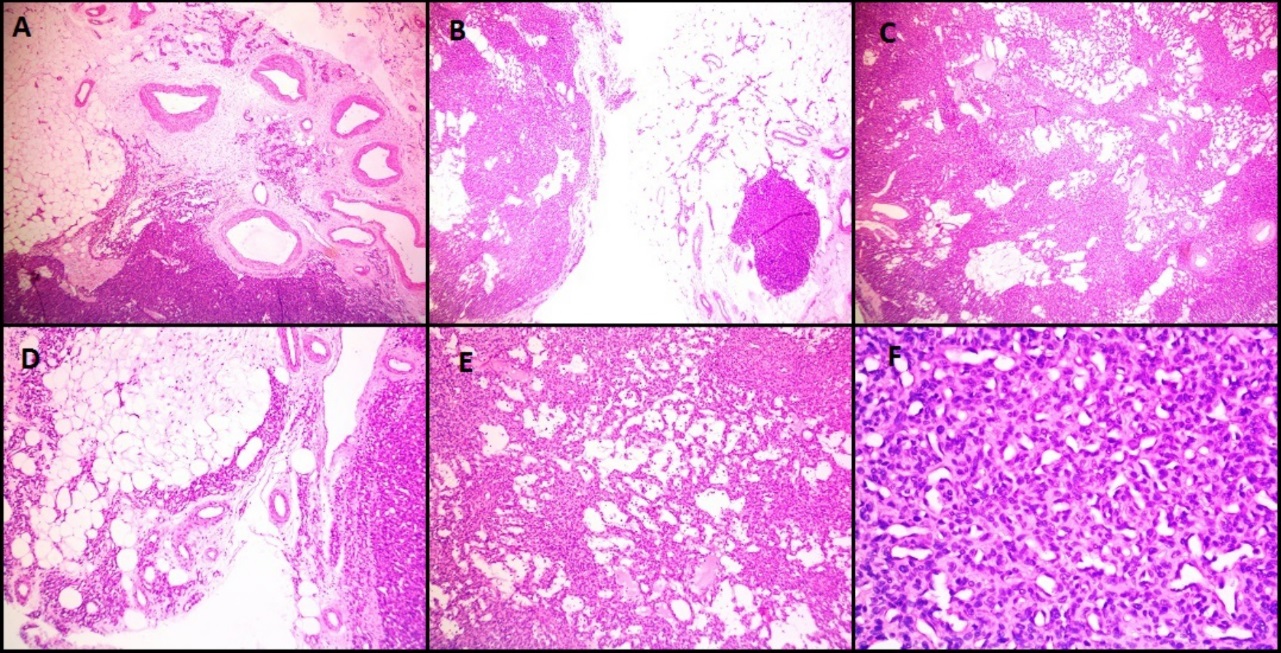
Figure 5
IHC images in case of glomangiomatosis (Case 4) showing expression of A): Vimentin, B): SMA, E): Calponin and F): H-Caldesmon. The tumor cells are negative for C): Desmin and D): CD34. CD34 stain highlights prominent vasculature. (A,B,C,D- 100X magnification, E, F- 400X magnification)
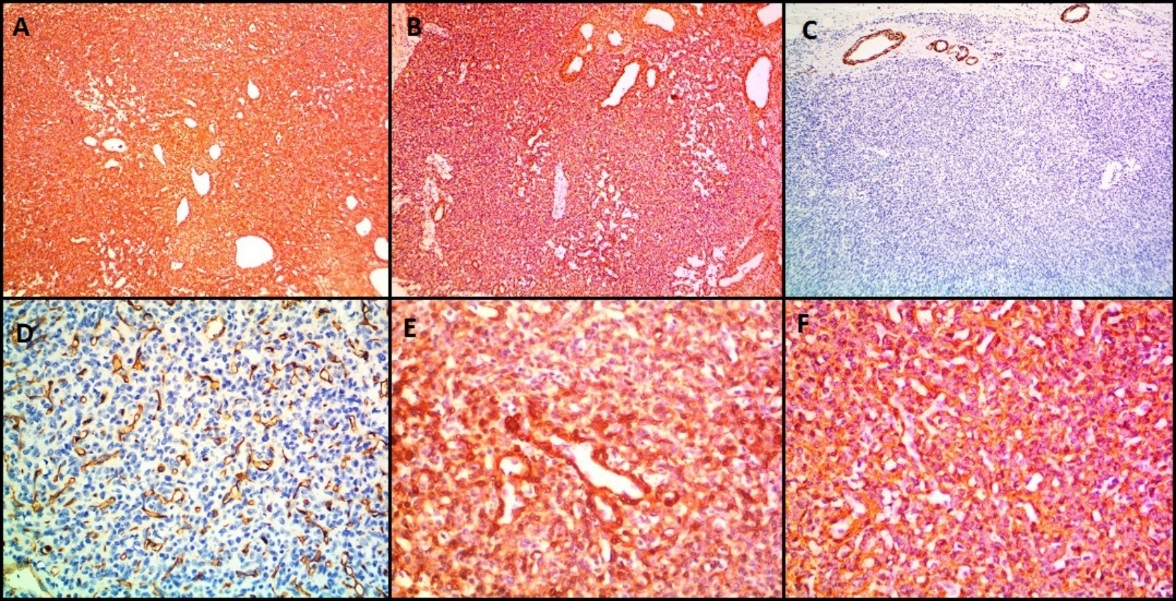
Figure 6
H&E images in case of malignant glomus tumor (Case 6) showing A): A subcutaneous lobulated tumor; B): composed of round epithelioid cells; C): with large hyperchromatic nuclei and clear cytoplasm. Black arrows point to the mitotic figures. (A-40X magnification, B: 100X magnification, C- 400 X magnification)
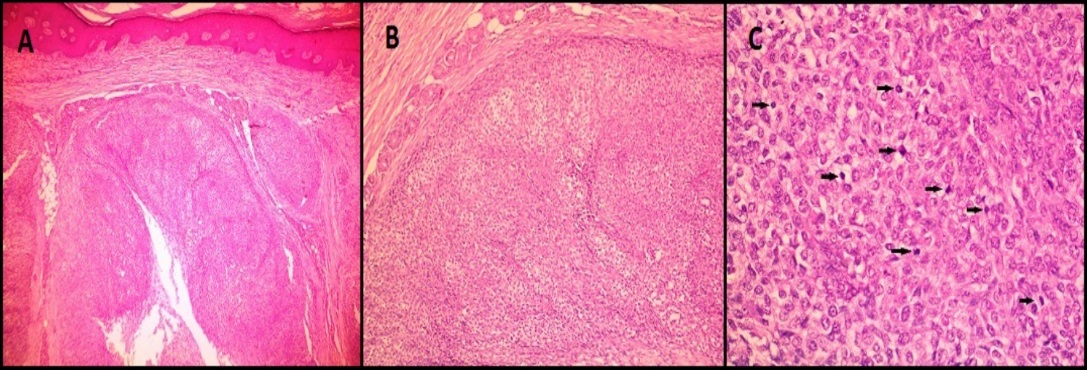
Figure 7
IHC images in a case of malignant glomus tumor (Case 6) showing expression of A): Vimentin, B): Smooth Muscle Actin (SMA), C) Calponin D): H-Caldesmon (A, C-100X magnification, B, D-400X magnification)
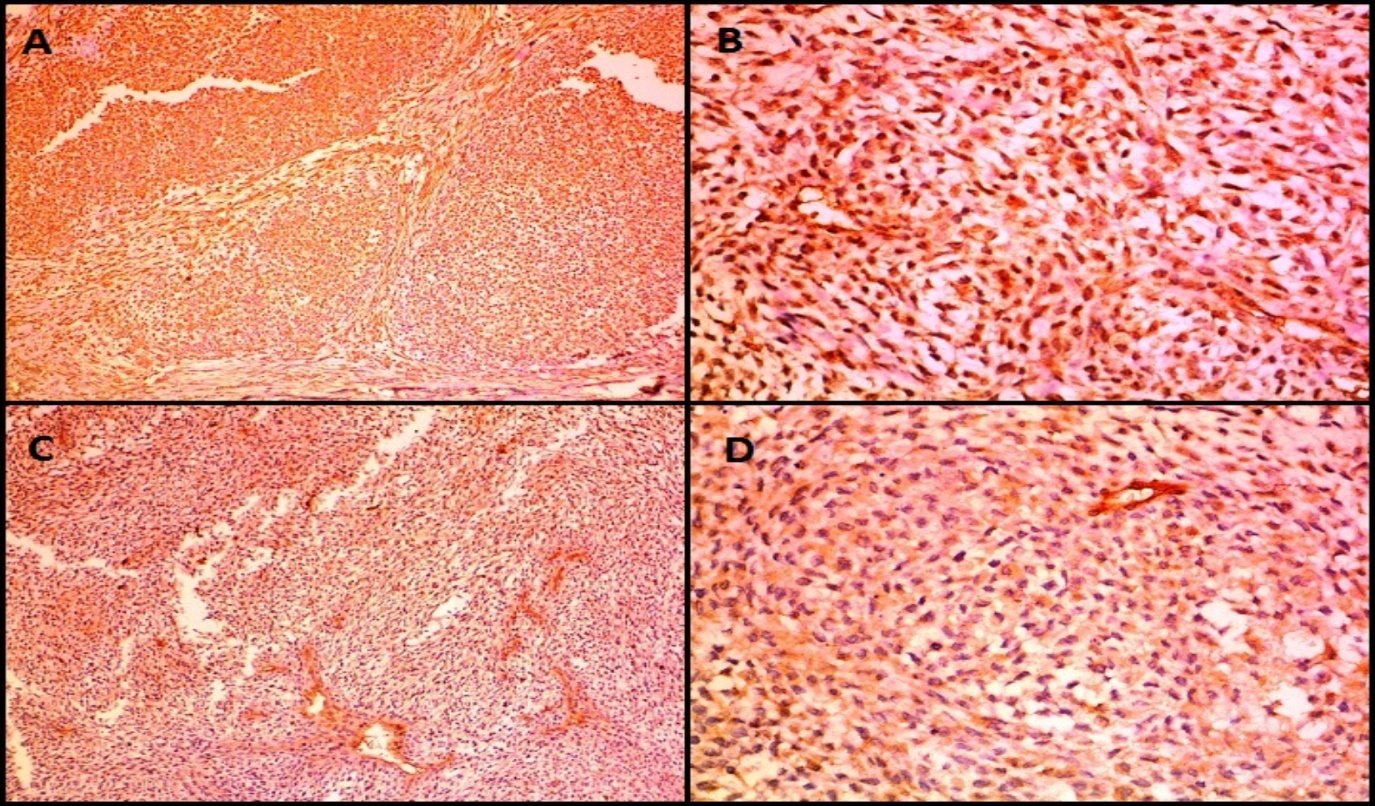
Figure 8
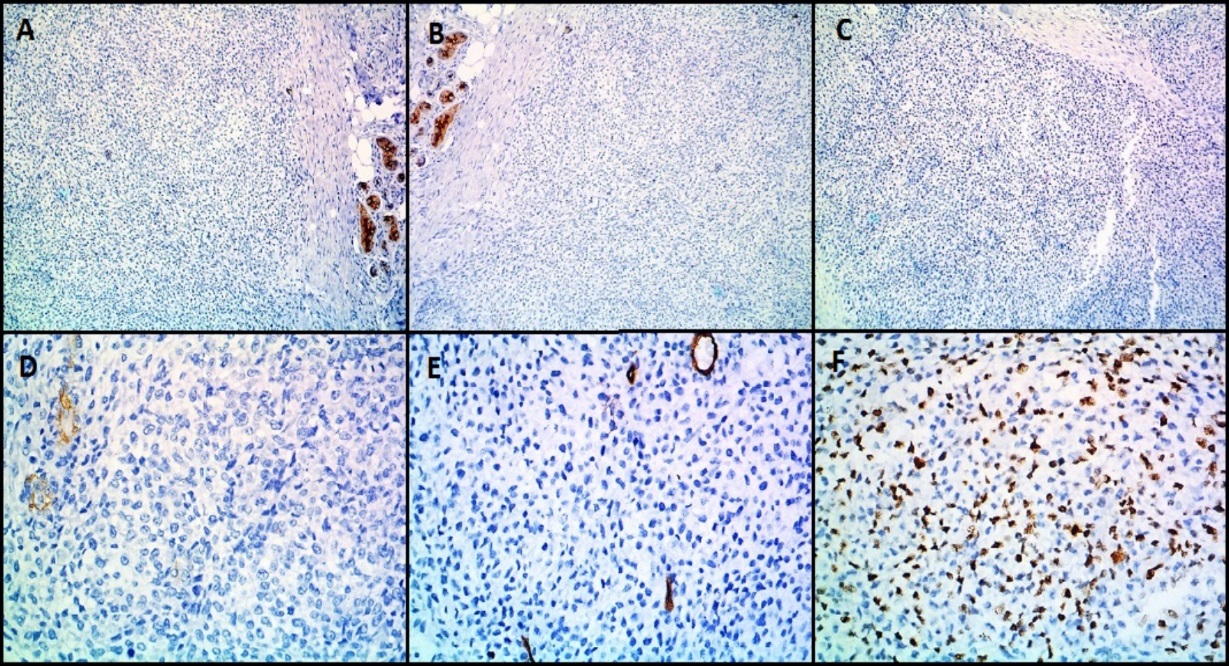
IHC images in case of malignant glomus tumor (Case 6) showing the tumor cells are negative for A): Cytokeratin (CK) B): Epithelial Membrane Antigen (EMA) C) S-100 D): Desmin E) CD34 F) Mib-1 shows increased Mib-1 proliferation index (A, B, C-100X magnification, D, E, F-400X magnification).
Multiplicity was observed in (2/9, 22.22 %) cases. Duration of symptoms till diagnosis showed a wide range from one month to nine years (median of three months). Preoperative diagnosis by imaging [Magnetic Resonance Imaging (MRI) and Computerized Tomography (CT) scan] were performed in only two cases (2/9, 22.22%). The size of the lesions in our series ranged from 0.3cm to 4.8 cm (mean 2.1±1.5cm). Excision biopsy was the surgical procedure performed in all of our benign cases while the malignant case was treated with amputation of the finger. Out of the nine cases, eight were benign (8/9, 88.89%) and one was malignant (1/9, 11.11%). Of the eight benign cases, two were glomangiomatosis (2/8, 25% benign cases). One of these two cases (case no. 8) had a simultaneous glomus tumor on the index finger and glomangiomyoma on the middle finger.
Discussion
Nearly 75% of glomus tumors occur in the upper extremities and the most common location is subungual.5, 8 However, only four cases of this series (4/9, 44.44% ) showed subungual location. But, when just the digital lesions in our case series are taken into account, 4 out of 5 that is 80% digital lesions were found in the subungual region.
The etiology of glomus tumor is currently not known. However, it may be associated with inheritance or trauma. Literature suggests that weakness in the structure of the glomus body can elicit post-trauma reactive hypertrophy. Truncating mutations in the glomulin gene (encodes protein 68-kDa with unknown function) which is linked to chromosome 1p21-22 were associated with a familial variant of glomus tumor.2
Awareness is essential for early diagnosis of this lesion as it is an extremely painful lesion and pain is out of proportion to the size of the lesion. The duration of symptoms before an accurate diagnosis is established is remarkably prolonged.8 In our case series, the duration of symptoms till the establishment of diagnosis showed a wide range from one month to nine years with a median of three months. In the case series by Fazwi et al5 average duration of time to diagnosis was 3.3 years with the longest being 10 years. Kumar et al8 showed an average duration of 2.3 years which was similar to our study but the longest duration was 5.6 years.
Review literature by Proietti et al 4 for shoulder glomus tumors states that in extra-digital areas, a subcutaneously located tumor is not diagnosed early even by palpation (duration of extra-digital locations can even extend up to 20 years before a diagnosis is made). At such times early imaging modality can come to rescue. Ultrasonography can locate the lesion but Magnetic Resonance Imaging (MRI) can give more details of the lesion and its relation with adjacent structures. Thus, MRI is the first investigation of choice. Typical MRI characteristics of glomus tumors on T1-weighted images are low signal intensity, T2-weighted images show marked hyperintensity, and after gadolinium injection, there is an enhancement on T1-weighted images.9 In our series, MRI or computed tomography (CT) scan had diagnostic utility in only 2/9, 22.22% of cases while in the rest of the other cases, histopathology was the primary diagnostic modality.
Glomus tumors are notorious for posing a ‘diagnostic dilemma’. Even though these tumors can be cured by surgical excision, clinical suspicion is low due to the rarity of this tumor and the usually small size and deep location of the lesion, resulting in delayed biopsies to reach an accurate diagnosis.8, 10 So, better vigilance of diagnostic clues and comprehension of morphology are required for timely diagnosis of this tumor. To solve this problem, Tang et al 11 in their article described a simplified algorithm-based approach to diagnose glomus tumors which is worth reading.
This tumor commonly presents with a clinical triad of paroxysmal pain, pinpoint tenderness and cold hypersensitivity.5, 12 In our case series all of the nine (9/9, 100%) cases presented with painful swellings. There were two cases which presented with multiplicity. Glomus tumors with multiplicity can have painful and painless lesions. Slepyan et al13 demonstrated that painless lesions have immature and dilated Sucquet Hoyer canals (afferent artery) as demonstrated by lack of epithelioid cell hyperplasia. This could explain why there is no pain in such cases since there is less pressure due to dilatation to cause any discomfort.
The size of the lesions in our series ranged from 0.3cm to 4.8 cm (mean size 2.1±1.5cm). Kumar et al8 showed a mean size of 0.8cm (0.32 to 1.62cm). Gombos et al14 in their review stated that benign solitary lesions usually are less than 1.0 cm while multiple tumors range from 0.1 to 0.3 cm in diameter but literature does report even 3.0 cm lesions.
Our cases showed a wide age range from 19 years to 78 years (mean age 37.67± 17.5 years). In a study by Kumar et al8 the mean age was 49 years while in a study by Fazwi et al5 the mean age was 49.6 years [age range 17-74 years].
Our case series showed male preponderance (Male-female ratio was 1.25:1). Female preponderance was noted in a study by Fazwi et al5 (female-to-male ratio of 1.3:1) and a study by Kumar et al 8 (female to male ratio 3.4:1).
Out of nine cases of our study, four (44.44%) were extra-digital locations (foot, suprapatellar and ankle). All our cases with extra-digital locations were males. Five out of nine cases (5/9, 55.56%) showed digital location (Table 1). Digital locations were found in both genders. However, all our subungual location cases were found in females. Fazwi et al5 also demonstrated (5/7, 71%) extra-digital glomus tumors occurred in men. Gombos et al14 in their review stated that subungual location was more prevalent in females while other loci did not show any sex predilection.
Maximum cases in our series 5/9, 55.56% showed left laterality while 4/9, 44.44% showed right laterality. Fazwi et al5 also demonstrated maximum left laterality in their case series.
The usual gross presentation of glomus tumors is deep red-purplish or bluish-discoloured soft mass. 5 Grossly, all our cases except for the malignant one showed externally skin-covered and congested lesions which on the cut section revealed an encapsulated tumor with light brown appearance. The malignant lesion showed ulcerated skin with a nodular myxoid appearance.
This tumor is histologically composed of glomus cells, blood vessels and smooth muscle cells. This forms the basis for categorizing glomus tumors into the following three types i) glomangiomas- with abundant blood vessels; ii) solid glomus tumors, comprising of glomus cells; and iii) glomangiomyomas showing smooth muscles predominantly. 15
All the benign glomus tumor cases exhibited typical morphology with small round to oval monomorphic glomus cells. Figure 1 shows microscopy images and Figure 2, Figure 3 show IHC images of case 8 (benign case).
Two of our cases (2/9, 22.22%) were diagnosed as glomangiomatosis. Both these cases showed extra-digital locations (ankle and foot) and one of them was multifocal.
Microscopically, both these lesions showed multiple diffusely infiltrating vessels of varying calibres. Small nodular masses were composed of uniform small round to oval epithelioid cells with monomorphic round nuclei and scanty eosinophilic cytoplasm. These tumor cells were arranged around the open vascular lumen and investing the vessels. No nuclear atypia, necrosis or mitoses seen. Figure 4 shows microscopy images and Figure 5 shows IHC images of case 4 (glomangiomatosis case).
Immunohistochemistry was done in all nine cases (Table 1). All nine cases expressed Vimentin, SMA and H-caldesmon. Out of nine cases, eight cases expressed Calponin and six cases expressed Desmin. The malignant case (case 6) did not express Desmin. CD34 (done in one of the glomangiomatosis) marked the vascular endothelium but perithelial tumor cells were negative.
Usually on Immunohistochemistry the tumor cells of glomus tumor show immunopositivity to Smooth Muscle Antigen (SMA), Vimentin and Type IV Collagen and are typically negative for epithelial markers. Immunoreactivity may also be noted for Desmin, Caldesmon and Calponin. Hemangiomas can be distinguished by the use of endothelial markers like CD34 which highlight its vascular network. However, few of these may co-express CD34 and myogenic markers.16
It is extremely rare to witness the malignant transformation of glomus tumor. Folpe et al 17 laid down the following diagnostic criteria for malignant glomus tumor: I) Size of more than two cm and deep location; II) Presence of atypical mitotic figures; or III) Combination of moderate to high nuclear grade and mitotic activity (5/50 high power fields).
One of our cases (1/9, 11.11%) was a malignant glomus tumor. The patient presented with painful swelling on the right ring finger for two months. The clinical diagnosis was of a skin adnexal tumor. A frozen section of incisional biopsy was received for primary diagnosis which was reported as a malignant tumor. Further, we received a specimen of amputation of the right ring finger for routine histopathology. Grossly, this specimen showed skin ulceration with underlying nodular and myxoid swelling with 3cm as the largest dimension.
Microscopically (Figure 6), the tumor was composed of spindle or epithelioid cells and plasmacytoid cells arranged in a lobular pattern and having clear cytoplasm. The mitotic count was 7/10hpf. Focal tumor necrosis was noted. Moderate nuclear atypia was seen. Differentials considered on H&E (Hematoxylin and Eosin stain) stained slides were malignant skin adnexal tumor, malignant glomus tumor, and malignant myoepithelioma. On immunohistochemistry (IHC) (Figure 7, Figure 8), the tumor cells expressed Vimentin, SMA, Calponin and H-caldesmon and were negative for Cytokeratin (CK), Epithelial Membrane Antigen (EMA), Desmin, CD34, S100, Myo-D1, p63 and CD99. Mib 1 proliferation index was 60%. A diagnosis of malignant glomus tumor was thus confirmed by IHC.
An extensive review of literature revealed at least 14 articles5, 8, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 documenting more than ten cases of glomus tumors of which five were reported in the Indian population.8, 20, 22, 24, 27 These literatures provide valuable insights into the characteristics of glomus tumors.
The treatment of glomus tumors is by surgical excision.29 All of our benign cases underwent surgical excision. The malignant case was treated with amputation of ring finger.
Literature reports a recurrence rate of 3 to 33% which can be most commonly associated with incomplete excision of the tumor.30
Kumar et al8 aptly suggested some learning points of glomus tumors which are as follows:
Glomus tumors usually present with the clinical triad of pain, pinpoint tenderness and cold hypersensitivity.
A small-sized painful swelling in the digits should always be evaluated for glomus tumors.
For extra-digital locations, painful lesions which demonstrate tenderness out of proportion to size of lesion should raise a possibility of glomus tumors.
As far as imaging is concerned, MRI is the investigation of choice. Histopathology is confirmatory and complete surgical excision is curative with low risk of recurrence.
Despite the interesting insights gained, this study has certain limitations. The small size of nine cases limits the generalizability of the findings to a larger population. Also, the retrospective approach may have introduced certain biases.
Conclusion
Diagnosis of glomus tumors requires a high index of clinical suspicion for rightly directing the appropriate investigations, especially in extra-digital locations. MRI is the investigation of choice for imaging. Early biopsy with histomorphological analysis showing uniform round cells with eosinophilic cytoplasm, centrally placed nuclei around vascular channels, and positivity for Vimentin, SMA and H-Caldesmon can confirm glomus tumors in time. These tumors are notoriously painful, may manifest with multiplicity and, although rare, malignancy should be evaluated histomorphologically along with the Mib-1 proliferation index. Surgical excision is curative however recurrence may occur if excision is incomplete.
Thus, by offering valuable insights into this rare neoplasm's characteristics and diagnostic strategies, this study hopes to improve diagnostic accuracy and patient care, thereby reducing suffering associated with delayed diagnosis.
