- Visibility 1k Views
- Downloads 64 Downloads
- Permissions
- DOI 10.18231/j.ijced.2025.002
-
CrossMark
- Citation
Nanocarrier-Mediated dermal drug delivery for skin disorders
Abstract
Skin diseases have a profound effect on the overall well-being of affected individuals, often associated with physical and mental anguish as well as social stigma. Traditional methods of treatment lack effectiveness in dealing with skin disorders and suffer from several limitations such as long treatment duration and high incidence of systemic side effects. Nanocarrier-based dermal drug delivery platforms help mitigate several of these limitations due to their site-specific delivery and superior retention at the target site, leading to minimization of off-target adverse events. Additionally, they bestow several theranostic advantages due to improved skin penetration as well as better control over drug release rates. This article advocates for the adoption of nanocarrier-based dermal drug delivery strategies as a solution to the shortcomings of conventional methods offering more effective, targeted, and patient-friendly treatments for several skin disorders such as psoriasis, atopic dermatitis and skin cancer.
Introduction
The skin forms as a massive protective barrier of our physique, covering approximately an astounding twenty square feet, wrapping the entire internal ecosystem of organs, bones, and muscles, while maintaining moisture balance and thermal homeostasis. Like every organ, the skin too is prone to dysregulation and disease. Disorders of the skin have genetic, metabolic, inflammatory, immune-system related or malignant origins and manifest as pathophysiological changes in the upper dermal layers or affect the deeper subcutaneous or systemic compartments.
While the healthy skin offers several advantages for topical administration over other routes, the plethora of damaging changes caused to the skin during disease makes topical treatment of these diseases difficult and challenging. Conventional topical dosage forms suffer from poor efficacy, long treatment regimens, condition relapses and systemic side effects due to nonspecific drug delivery. To overcome the major pitfalls opposing site-specific drug delivery and penetration through the skin barriers, nanocarrier-mediation and delivery of drugs have been widely explored with excellent degrees of success. The present review describes the design principles and applications of nanoparticulate dermal drug delivery systems exploited for a spectrum of skin disorders such as psoriasis, atopic dermatitis and skin cancer.
Drawbacks of conventional therapy
Several issues plague topical treatment approaches. Damaged, inflamed skin encountered in dermal disorders affects drug permeability through the stratum corneum. Formation of dense, scaly plaques as seen in diseases like psoriasis, low ceramide levels, and high cholesterol levels and lack of loss of normal skin hydration prevents the drugs from deep skin penetration. Failure to pass through tightly bound epithelial and endothelial layers, difficulty in reaching the intracellular arena in sufficient concentration and achieve tissue-specific targeted drug delivery further worsen the overall efficacy of treatment. The older systemic agents typically have issues with poor oral bioavailability, hepatic pass metabolism, poor skin distribution, rapid clearance as well as a higher incidence of toxic side effects.[1]
Nanotechnological design principles
An array of nanotech-based methods have been designed to tackle the challenges of conventional treatment. Each of these approaches offers unique advantages, leveraging the principles of nanotechnology to improve drug delivery and efficacy while minimizing side effects.
The skin provides a protective barricade to the external bios, making it extremely challenging to permeate by most compounds. Keratinocyte hyperproliferation as observed in certain diseases further aggravates the permeability issue. Nanotechnological interventions help circumvent the challenges of poor penetration. The combined effect of small size and high surface area of the nanoparticles enables longer residence time on the skin and also promotes deeper penetration.
Drugs that are incorporated into nanocarriers are deposited on the skin for prolonged time periods and have longer shelf lives. The films that are produced create a concentration gradient that causes the drug to diffuse into the skin layers, thereby giving better control in drug release rates.[2]
Solid phase nanocarriers, liquid phase nanocarriers, and liquid crystalline phase nanocarriers are the three main classes of the carriers that have been used for treatment of dermal diseases.[3] Polymeric nanoparticles establish a favorable concentration gradient across the skin surface that results in enhanced skin permeation. They also provide a sustained rate of release at the delivery site, prolonging the therapeutic effect. Lipid-based nanocarriers adhere effectively to the skin surface and establish close contact with the stratum corneum, facilitating better penetration and efficacy of drugs. Nanostructured lipid carriers (NLCs) are amorphous with structural imperfections within the structural matrix, allowing for better drug loading as well as retention during storage. They also have the ability to retain moisture via formation of an occlusive layer, leading to extended retention times in skin layers and controlled release. Nanoemulsions have been found to be particularly beneficial for treatment of psoriasis due to their ability to permeate the diseased skin plaques via manipulation of skin lipids, enhancing the dermal bioavailability of drugs.
Vesicular systems like ethosomes and niosomes contain edge activators and surfactants that weaken phospholipid bonds, making them ultra-deformable. The resultant decrease in vesicular rupture and leakiness improves the efficiency of drug delivery. Ethosomes contain ethanol, which interacts with polar lipid heads, enhancing fluidity and decreasing density, permitting easy passage of drugs. Fusion with skin lipids facilitates drug release and deep skin penetration that is particularly useful for lipophilic drugs. Niosomic vesicles exhibit absorption and fusion on the skin surface that creates an interfacial concentration gradient with better drug permeation across the stratum corneum. They also possess permeation enhancer properties, further facilitating drug transfer. Figure 1 gives a schematic representation of the design principles exploited in nanotechnological approaches to dermal delivery of drugs for various skin diseases.
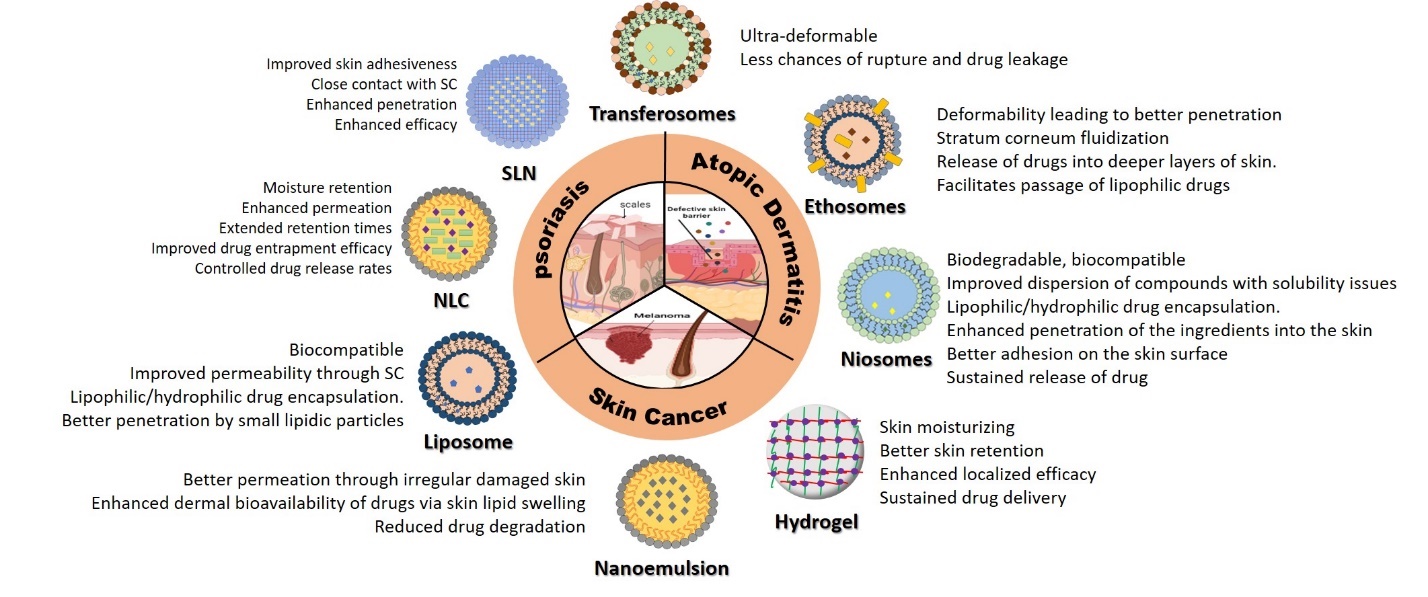
Psoriasis
Psoriasis is a long-standing immune skin disorder plagued by itchy, scaly, and flaky skin patches, inflammation, and painful, disfiguring lesions. It has an extremely complex pathological landscape and is thought to be a genetic condition succumbing to environmental triggers. The disease manifests in the form of inflamed skin plaques, pustules, red papules, silvery scales and widespread inflammation and exfoliation of the skin. [4] Treatment regimens include anti-inflammatory and immunosuppressive drugs such as steroids, salicylic acid, vitamin D3, methotrexate, cyclosporin, retinoids and acitretin. Conventionally, topical therapy is given for mild symptoms and systemic therapeutic agents are administered in moderate to severe disease conditions. Various nanocarriers have been explored for their enhanced potential in the therapeutics of psoriasis. These include nanostructured lipid carriers (NLC), polymeric nanoparticles, solid lipid nanoparticles (SLN), liposomes, nanoemulsions, ethosomes, transferosomes and niosomes.
Polymeric nanoparticles
Drug compounds can be contained as solid particles or particulate dispersions and formulated via encapsulation, adsorption or dispersion in a suitable polymer matrix. Polymeric nanoparticles are generally constructed using biocompatible and biodegradable polymers, and induce enhancement in drug bioavailability, drug release along with a reduction in toxicity and undesirable effects. Particle typically adopt sizes ranging from 10 to 1000 nm. These polymeric nanoparticles can encapsulate hydrophilic medications, making them potential carriers for the delivery of psoriasis drugs across the skin. Additionally, they permit regulated release of medication at the delivery site by establishment of a concentration gradient across the skin layers.
Curcumin holds promise for treating psoriasis. However, its limitations in solubility and skin penetration hinder its effectiveness. Utilizing nanoparticle-gel systems offers a promising approach to address these challenges and improve the therapeutic outcomes of curcumin in psoriasis treatment. Mao et al. created skin-permeating nanoparticles with the amphiphilic RRR-tocopheryl succinate-grafted-polylysine conjugate (VES-g--PLL) as polymer. They found that the particles had a small hydrodynamic diameter and high zeta potential, which made it simple for the particles to penetrate the skin. The formulation afforded improved anti-psoriatic activity by effectively inhibiting the expression of inflammatory cytokines like IL-6, TNF-, and NF-B.[5] The authors attributed the enhancement in efficacy to the deeper and improved skin permeation and translocation of curcumin across the dermal layers due to the formation of an occlusive fine gel layer on the psoriatic skin. This increased the hydration level as well as contact time of the formulation on the skin.
Drugs with high molecular weights and complex cyclic structures face additional penetration challenges through the damaged stratum corneum of psoriatic skin. Moreover, long-term administration causes systemic toxicity effects such as nephrotoxicity, hypertension, hepatitis, and hyperkalemia.
Badilli et al. investigated the effectiveness of Clobetasol 17-propionate loaded PLGA nanoparticles in the treatment of psoriasis. These nanoparticles offered a delayed but prolonged release profile of the drug into the skin, improving the drug's stability and reducing systemic side effects significantly compared to the conventional formulations. [6]
Solid lipid nanoparticles (SLN)
Solid lipid nanoparticles (SLNs) are lipid-based vesicular systems that have grown in popularity because of their capacity for controlled, site-specific drug release. Their small size and ability to form lipid films on the skin allows for better retention and deeper penetration into the stratum corneum along with enhanced skin hydration.
According to Pradhan et al., triamcinolone formulated as topical SLNs for psoriasis offered better skin layer permeation as well as a prolonged drug release profile without significant systemic absorption. They discovered that keratinocyte growth abruptly decreased, which led to a reduction in epidermis thickness. The benefits over traditional creams were reduced skin irritability and restricted distribution to the dermis and epidermis. [7]
Tacrolimus was formulated as SLN in hydrogel, according to Wang et al., and was superior to commercial formulations in terms of localization and penetration into the skin's layers. Additionally, they predicted that when used topically, the SLNs would have excellent release properties and little systemic absorption. [8]
Nanostructured lipid carriers (NLC
Nanostructured lipid carriers (NLCs) are second generation lipid nanoparticles with sizes in the nanometer range that allows for deep penetration into the skin. NLCs have the capacity to shield drug molecules from oxidative, chemical, and physical deterioration.
When applied topically to a psoriatic plaque model induced by imiquimod, Sathe et al. demonstrated the easy applicability dithranol NLC gels along with improved efficacy over conventional formulations. They provided explanations for several characteristics that related to the severity of the disease being less severe, such as a lower PASI score and lower levels of key cytokines like IL-17, 22, and 23, which are involved in the pathogenesis of psoriasis and enhance the anti-psoriatic activity of the substance. [9]
It was found that adding methotrexate-loaded NLC to a hydrogel for the treatment of psoriasis increased the compound's antipsoriatic efficacy and decreased the associated side effects. Additionally, they noted that the drug was eliminated from the body more gradually than with standard preparations. [10]
According to Avasatthi et al.'s research, methotrexate-loaded NLC was more effective than methotrexate conventional gel at treating psoriasis in mice by resulting in a lower PASI score. Pinto et al. reported greater skin permeation in Methotrexate-loaded NLC in psoriasis, as opposed to conventional formulations. The NLC displayed a flux of 0.88 g/cm2/h, which was significantly higher (p0.001) than the free drug's flux of 0.59 g/cm2/h and suggests the potential of NLC in the delivery of drugs for psoriasis. [11]
Tacrolimus was found to penetrate the skin 1.64 times deeper when it was formulated as NLCs for the treatment of psoriasis as reported by Nam et al. This suggests an excellent system to improve tacrolimus delivery to the skin. [12]
Improved efficacy and compatibility of two medications with different polarities such as calcipotriol and methotrexate into NLCs was demonstrated. Methotrexate from NLC improved skin permeation by 2.4 to 4.4 times when compared to the control, but calcipotriol did not show a significant improvement. In comparison to the traditional preparations of both medications, which are commonly used to treat psoriasis, there was also a decrease in side effects. [13]
Liposomes
Liposomes are spherical phospholipid vesicles with ability to deliver lipophilic as well as hydrophilic drugs and are frequently applied topically for various dermatological disorders, including atopic dermatitis, psoriasis and vitiligo. They are lipidic and small, making it possible to deliver medications to specific skin regions.
In an imiquimod-induced plaque psoriasis model, Walunj et al. used cyclosporine-loaded cationic liposomes for the psoriasis treatment. Topical liposomal preparations rapidly reduced psoriasis symptoms with concurrent reduction of cytokines that control the condition, including IL-17, IL-23, and TNF-. This was an improvement over traditional formulations in terms of efficacy. [14]
Lai et al. demonstrated the effectiveness of yolk lecithin liposomes in a mouse model of imiquimod induced psoriatic plaque. It was found that stronger skin barriers against psoriasis-inducing substances like imiquimod worked more effectively. Additionally, they showed that key psoriasis regulatory cytokines like IL-23 and TNF- were reduced, which lessened the disease's symptoms. In order to assess the effectiveness of cyclosporine-loaded liposomal formulations. [15]
Kumar et al. conducted a single-centered, randomized clinical trial practice with 38 patients who had chronic plaque psoriasis. They came to the conclusion that liposomal gel was more effective at treating psoriasis than conventional cream with the same concentration of cyclosporine. [16]
Nanoemulsions
The size of a nanoemulsion globule is 50–200 nm. During the treatment of psoriasis, they are used as a crucial tool to increase the permeability of drugs into the dermal layers, providing benefits like highest entrapment effect, fewer adverse effects, better skin permeation, reduced skin irritation, and a slow release of the drug incorporated. [17]
Rajitha et al. used an emulsification technique to study chaulmoogra oil-based methotrexate nano emulsions for psoriasis treatment with better drug permeation as well as improved retention into the skin layers. The anti-psoriatic activity of the drug in an in vivo model was improved, and there was less drug accumulation in serum and tissues. [18]
The anti-psoriatic activity of tacrolimus nanoemulsion was assessed by Sahu et al. by estimating the drug's bioavailability in skin, and reported a 4.33 fold in in vitro studies. Studies conducted in animals showed that the drug's anti-psoriatic activity was enhanced because there were fewer cytokines present than in the commercial formulation. [19]
In order to increase the drug's solubility, Musa et al. created cyclosporine-loaded nano emulsions of virgin coconut oil and nutmeg oil for psoriasis. Studies conducted in vitro with the formulation showed increased efficacy of the formulation over the standard products. [20]
According to Alam et al., clobetasol propionate significantly increased its anti-inflammatory activity when it was made into nanoemulsions and applied topically to treat psoriasis. Additionally, a notable reduction in skin irritation compared to the drug's traditional cream was noted. [21]
Deformable liposomes
Although liposomes have been extensively used to deliver various medications for psoriasis treatment, they have limited applicability in transdermal delivery as they are capable of penetrating only the superficial stratum corneum. Deformable drug carriers facilitate transport of drugs across the skin without disrupting the normal functioning of the epidermal lipid layers. Deformable liposomes have been used to dermal drug delivery and address issues brought on by liposomes' inadequate topical absorption. They were first described as a new class of vesicular carriers with improved flexibility or elasticity by Cevc and Blume. [22]
Ethosomes, transferosomes, and menthosomes were the vesicles that manifested as deformable liposomes. Compared to liposomes, all of them are more effective at crossing the stratum corneum. [23]
According to Srisuk et al., when used to treat psoriasis, methotrexate-loaded deformable liposomes provided improved skin permeability and better accumulation in the epidermal and dermal layers. [24]
When compared to the same drug's water solution, Trotta et al. found high skin permeability with methotrexate-loaded deformable liposomes for the treatment of psoriasis. According to the study, using deformable liposomes increased drug absorption through the skin by about 3 to 4 folds when compared to using regular liposomes, and it also increased dose accumulation in the skin by about 50%. [25]
Transferosomes
The second generation of elastic deformable lipid vesicle carriers, transferosomes were first introduced in the early 1990s. They are highly deformable unilamellar vesicles with diameters under 100 nm and are composed primarily of phospholipids with a preferred ratio of 85:15 to edge activators. Drugs are transported by transferosomes across the skin layers without the need for fluidization or stratum corneum lipid binding; instead, the vesicles are moved as non-fragmented bilayers across a barrier that is semipermeable, with hydro tactic as the driving force. [23]
However, the penetration continues to be totally unaffected by the partition coefficient and concentration gradient. El-Gizaway et al. reported the development of betamethasone dipropionate-loaded transferosomes for psoriasis that could deliver the medication more deeply into the dermal tissues and provided patients with plaque type psoriasis with good tolerability. The stability and anti-psoriatic activity were also improved compared to traditional formulations. [26] All information on the use of transferosome formulations for the treatment of psoriasis was compiled by Sala et al. [27]
When compared to traditional ointment, tacrolimus-loaded transferosome gel showed enhanced accumulation in the skin's dermis and epidermis, according to Lei et al. In comparison to the ointment, tacrolimus accumulated 3.8 and 4.2 times more in the epidermis and dermis, respectively, showing improved topical delivery in psoriasis. [28]
In their investigation of hydrocortisone loaded transferosomes, Cevc et al. discovered a 3 to 5-fold efficacy improvement over typical creams or lotions. Along with the increased therapeutic efficacy, a decrease in sensitivity and application frequency was also noted. Additionally, compared to conventional products, dexamethasone-containing transferosomes offered an increase of fourfold in the time of action, an improved risk-benefit ratio, and a reduced frequency of application. [29]
Ethosomes
The third generation of elastic deformable lipid vesicles includes ethosomes. They are primarily ethanolic vesicles composed of phospholipids and water. The amount of ethanol in the mixture can range from 20 to 45%, and it's used to increase the vesicles' fluidity as well as permeability. [30]
In order to treat psoriasis, Zhang et al. investigated the effects of adding hyaluronic acid to PG i.e curcumin-loaded propylene glycol-based ethosomes. This method had several benefits over plain ethosomes and propylene solutions, including decreased drug leakage, improved permeation, and increased retention up to 2.3 and 4 folds, respectively. After using the formulation, the levels of the main cytokines controlling the pathogenesis of psoriasis, including IL-17A, IL-17F, and IL-22, decreased. [31]
According to Zhang et al. topical psoralen ethosomes demonstrated enhanced drug permeation and retention in the skin's layers with 6.5-fold higher permeability than traditional tincture. The peak concentration and the AUC from the ethosomes were 3.37 and 2.34 times higher respectively. They also came to the conclusion that the drug had a better anti-psoriatic efficacy and was less toxic. [32]
According to Fan et al., tacrolimus-loaded ethosomes accumulated to a greater extent in the epidermal layers during psoriasis treatment and significantly improved epidermal penetration (p0.01) when compared to conventional formulations. [33]
Niosomes
Niosomes are microscopic lamellar vesicles made of non-ionic surfactants that are created when non-ionized surfactant and cholesterol are hydrated. By shielding the drug from the environment, they have reportedly improved therapeutic efficacy. They have advantages over traditional formulations because they are biodegradable, biocompatible, and nonimmunogenic by nature. [34]
Hashim et al. created niosomal gels of Acitretin to address issues with side effects and skin irritation that are present in conventional formulations. In comparison to the standard conditions, niosomal gels provided decreased the thickness of the epidermis and dermal irritability, while increasing orthokeratosis and overall drug activity. They also reported that the drug penetrated deeper into the skin with minimum absorption into the systemic circulation, proving its superior efficacy to traditional gels used in psoriasis. [35]
According to Priprem et al., niosomes of anthocyanin complexes offered sustained drug action during psoriasis treatment with anti-inflammatory activity comparable to fluocinolone acetonide with significant decrease in psoriatic skin lesions. [36]
According to Abdelbary et al., methotrexate loaded niosomal formulations showed improved drug penetration and accumulation when compared to the drug's straightforward conventional solution and was safe for topical treatment of psoriasis. Furthermore, methotrexate loaded niosomes were more effective in in-vivo studies at treating psoriasis than traditional formulations due to higher methotrexate deposition in skin layers. [37]
Atopic Dermatitis (AD)
Atopic dermatitis is a immunological skin disease with typical symptoms of chronic eczema, lesions and uncontrollable itching. Notable symptoms include varying degrees of erythema, skin scaling, and pruritis and dry stratum corneum causing intense itchiness. Traditional therapy seeks to alleviate itchiness and inflammation by administration of corticosteroids and calcineurin inhibitors, enhance skin hydration with moisturizers and emollients, and improve the barrier functionality of skin. The preferred route of administration is topical due to the failure of other routes in delivering an efficacious dose to the skin. However, conventional topical dosage forms also suffer from limited deep skin penetrability into the altered AD skin. Nanocarrier-mediated dermal delivery approaches are being researched in order to overcome the challenges integral to AD therapy. [38] Nanoencapsulation permits dose reduction, controlled drug delivery rates, site-specific drug delivery, lower side effect liability, improvement in drug solubility and enhanced skin permeation. Several nanocarriers have been explored for treatment of AD including polymeric nanoparticles, inorganic nanocarriers, lipid-based nanocarriers and hydrogel systems.
Polymeric nanoparticles
Polymeric nanoparticles are biocompatible, bioadhesive materials with several advantages such as controlled rates of drug delivery, high entrapment effectiveness, improved stability, and versatility for delivery of both hydrophobic and hydrophilic drugs. Hussain and colleagues investigated the coencapsulation of hydrocortisone and hydroxytryosol in chitosan nanoparticles. Compared to control groups, Atopic dermatitis mouse models injected with nanoformulations had a two-fold reduction in skin thickness with significant reduction in immune markers like IgE, histamine, T-helper cells (TH1/TH2), the prostaglandin E2, and endothelial growth factor. In a related study, NPs added to a QV cream as well as nanoparticles of chitosan loaded with active compounds were given to AD mouse models (NC/Nga). When compared to animals treated with cream-based formulation, only NPs resulted in significantly higher epidermal as well as dermal retention of hydrocortisone and hydroxytryosol. Up until the fourth week, the TEWL control of both formulations was comparable; however, in the two weeks that followed, the NPs outperformed the oil-based formulation. [39]
According to Rosado et al., polycaprolactone nanoparticles of hydrocortisone significantly reduced the drug's toxicity when used to treat psoriasis, showing improved drug release control and greater permeation into the skin layers. [40] The safety and tolerability evaluation of these nanoparticles in cream-based formulations demonstrated lack of irritation or any other untoward toxicities. [41]
The encapsulation of medications that target the skin immune system is another promising strategy for treating moderate to severe AD. For instance, the calcineurin inhibitor cyclosporine A have been shown to have been nano encapsulated with subsequent reduction in formation of pro-inflammatory cytokines like IL-2 necessary for T-cell activation pathways.
PLGA nanoparticle-loaded cyclosporine was studied by Badihi et al. to treat severe AD. The nanoparticles exhibited greater inhibition of proliferation of cells along with the exhaustion of CD8, CD25, and CD69 in comparison with nonencapsulated drugs while reducing the secretion of IL-2, according to in vitro anti-inflammatory efficacy using mouse splenocytes. [42]
In another study, the immune suppressant tacrolimus, in combination with unconventional bioactives like nicotinamide as an adjuvant were explored for the treatment of AD. These bioactives have anti-inflammatory and antimicrobial properties. The high molecular weight and hydrophobicity of tacrolimus was found to hinder the formulation of hydrophilic matrices, such as gels, and limit drug penetration into deeper skin layers. [43]
Inorganic nanocarriers
Inorganic nanomaterials are being widely explored for dermal delivery of drugs due to their inherent optical and super paramagnetic properties, biological compatibility and superior encapsulation efficiency. Despite the fact that many nano-based formulations are helpful in the treatment of skin disorders, they can also cause allergic reactions in patients with AD because of sensitive skin or an induced immune response. [44] An in-vivo study to evaluate the effect of concurrent exposure of polystyrene nanoparticles and mite antigen in a mouse model pointed towards better induction of skin lesions resembling AD with the combination as compared to mite antigen alone. This was accompanied by elevated IL-4 expression and lower interferon generation. The size of the nanoparticles affected how much more symptoms of AD were induced after exposure, with smaller nanoparticles producing more symptoms. [45] More recently, Kang et al. demonstrated a significant improvement in allergic reaction in NC/Nga mice using silver nanoparticles that was directly influenced by the size of the nanoparticles. Mast cell treatment with nano-sized silver nanoparticles resulted in elevated ROS generation, according to in vitro tests. Additionally, in comparison to animals subjected to only silver nanoparticles or mite allergen AD mouse models co-exposed to both together displayed higher intensity of skin lesions and an increase in the number of tryptase-positive mast cells, and increased IgE serum levels. [46] Animals injected with SiO2 nanoparticles showed increased IgE production. Ilves et al. investigated the effect of various-sized zinc oxide particles on damaged and allergic skin in BALB/c mouse models of AD. Zinc oxide nanoparticles penetrated the dermis and epidermis of damaged allergic skin as compared to larger moeities. Pro-inflammatory (IL-4, IL-13, IL33, IL-1, IL-6, TNF and anti-inflammatory cytokine IL-10 were all significantly reduced by ZnO nanoparticles. However, following being exposed to ZnO nanoparticles, there has been an increase in IgE production in serum, pointing towards an allergic artifact.
Lipid-based nanocarriers
Nanoformulations based on lipids are highly favorable for skin therapeutics. They can be manipulated to provide high entrapment efficiency for both polar as well as hydrophobic substances. Various lipid-based systems with promising outcomes, such as ethosomes, transfersomes, nanoemulsions, liposomes, and cycloethosomes have been developed for topical use.
Liposomes
Given their various compositions and incorporated molecules, liposomes have superior attributes for transdermal delivery system for the treatment of AD. Akhtar et al. assessed the ability of fluocinolone acetonide-loaded cycloethosomes to penetrate the body and increase drug solubility and encapsulation effectiveness. There were few morphological changes seen on the stratum corneum, according to the authors, which indicated that ethanol improved drug absorption into the skin layers, pointing towards the superior capabilities of ethosomes. [47]
Another study created ultraflexible vesicles to deliver cyclosporine A in an effort to get around the drug's extremely high molecular weight and lipophilicity restrictions. This was done in an effort to enable deep penetration of drugs into skin. Vesicles made with ethanol produced transfersomes that enhanced cyclosporin accumulation into the membrane. [48]
Advances in therapies for skin inflammatory disorders have explored siRNA for slowing down disease pathology and progression. Kang et al. developed tacrolimus-loaded pH-responsive lipid nanoparticles for enhanced the skin penetrability and drug accumulation. Ex-vivo permeation tests revealed greater skin penetration in comparison to the standard product. This method represented a significant advancement in the creation of a potential commercial product. [43]
Nanoemulsions
Lipid-based nanoemulsions provide an excellent platform as drug carriers due to the various opportunities it provides such as improvements in solubility, stability, skin bioavailability, hydration, elasticity and microbial resistance. Baspinar et al. reported the development of positively charged phytosphingosine nanoemulsions, which provided physical, chemical as well as colloidal stability to the formulation. Phytosphingosine is a ceramide precursor that stimulates the stratum corneum and aids in lipid regeneration and elasticity. Additionally, the positive charge enhanced skin adsorption. [49]
Espinoza et al. prepared castor oil-based pioglitazone nanoemulsions with PEG-8 caprylic glycerides, diethylene glycol monoethyl ether and propylene glycol as colloidal carriers. The formulations showed excellent skin penetration and anti-inflammatory effects, favorable skin retention as well as additional stratum corneum hydration. [50]
Hydrogels systems
Hydrogels are built as polymeric mesh matrices that have been chemically or physically cross-linked. If there is enough water present, they can swell. Interactions in polymeric chains caused by crosslinking due to formation of hydrogen bonds, ionic bonds along with other types of non-bonded connections mediated by hydrophobic bonding, or van der Waals interactions. A mediator agent is required for chemical crosslinking to encourage covalent bonds among polymeric chains. The hydrophilic groups that already exist in the polymeric backbone of hydrogels allow them to absorb water. On the contrary, polymer chain crosslinking results in resistance to dissolution. [51] Hydrogels of natural and synthetic origin have been thoroughly explored for biomedical applications due to their attributes of biodegradability, biological compatibility, porosity, mechanical strength, and low elastic surface tension.
Lee et al. developed a prednisolone-loaded alginate/polyvinyl alcohol and tested them in AD mouse models. [52] In comparison to untreated animals, those given the hydrogel showed a significant reduction in blood levels of IgE and mast cells.
A thermoreversible hydrogel based on poloxamer (P407) was topically tested by Napimoga et al. for delivery of 15 deoxy Δ12,14 PGJ2 (15d PGJ2) in AD mouse models. In comparison to control animals, animals given hydrogel formulations had significantly lower levels of serum IgE and skin invasion of mast cells. Additionally, ROR-g10 and TNF-positive cell expression significantly decreased in animals given hydrogel formulations. With hydrogel formulations, the clinical AD score and skin moisturization both improved, and ear thickness was reduced. Hydrogel formulations also significantly reduced mast cell infiltration on animals receiving treatment. [53]
Polymeric hydrogels are preferred as matrices for incorporating nanocarriers because of their high viscosity and ability to thicken liquids. Additional advantages include better skin retention, sustained drug delivery and improved therapeutic efficacy for localized applications.
As a pharmacological treatment for AD, corticoids have been widely used. However, they are plagued by undesirable side effects, including immune suppression, dermal atrophy, and skin irritation. Delivery of these drugs with the aid of nanocarriers has helped mitigate this problem, Steroidal drugs such as betamethasone valerate or diflucortolone valerate have been encapsulated in lecithin- chitosan nanoparticles and soybean phosphatidylcholine-based liposomes before being dispersed in 2% chitosan hydrogel by Eroglu et al. [54] In contrast to commercially available formulations, liposome-chitosan hydrogel exhibited better skin retention in the upper layers of the stratum corneum and epidermis due to their similar mean sizes, with better anti-inflammatory effects as compared to the nanoparticle hydrogels. Both systems were more effective than commercial creams that contained ten times less corticoid, which improved the formulations' dermal safety.
Skin Cancers
Nanocarrier-based delivery systems deliver promising solutions to counter limitations of conventional chemotherapeutics in treating cancer. By encapsulating anticancer agents within nanocarriers, researchers aim to enhance drug delivery efficiency while minimizing systemic toxicity. Nanocarriers have the ability to improve the bioavailability of water-insoluble compounds, which traditionally exhibit poor absorption and limited therapeutic efficacy. Through controlled release mechanisms, nanocarriers can sustain drug concentrations at the target site, optimizing therapeutic outcomes. Moreover, the tunable properties of nanocarriers allow for customization to specific cancer types and patient needs. By incorporating targeting ligands onto the surface of nanocarriers, selective delivery to cancer cells can be achieved, reducing off-target effects and enhancing treatment efficacy. Additionally, the integration of theranostic agents enables simultaneous diagnosis and therapy, facilitating personalized medicine approaches. Among the various nanoparticulate systems explored for skin cancer treatment, liposomes, metallic nanoparticles, and lipid-based nanocarriers stand out for their biocompatibility, versatility, and tunable properties. Liposomes, for instance, offer a lipid bilayer structure ideal for encapsulating both hydrophobic and hydrophilic drugs, while metallic nanoparticles provide unique optical and magnetic properties suitable for imaging and targeted therapy applications.
Liposomes
Liposomes are dual-cored small vesicles with phospholipids engulfing a hydrophilic core. They have cholesterol and environmentally friendly phospholipids as their primary constituents, making them highly effective as transporters of bioactive moieties through the skin. Numerous qualities, such as favorable tissue compatibility, half-lives, penetrability occlusivity and, low immunogenicity are responsible for the rising demand for liposomal candidates. Due to the target-specific release of liposomes, researchers have been able to combine multiple drugs to produce desired cytotoxic action and a decrease in adverse events.
Calinni et al. developed ultra deformable liposomes containing Vismodegib and demonstrated their low elastic modulus attributed to higher deformability as compared to its non-conjugate. Such preparations maintain the sanctity of the drug delivery system without affecting the penetrability of the drug. [55]
Quercetin and reseveratrol were formulated into liposomes in a study by Caddeo and colleagues. The formulation had favorable attributes of particle size, zeta potential and stability index and displayed significant antioxidant and anti-inflammatory synergistic potential. [56]
Gold nanoparticles
Gold nanoparticles have gained popularity in the biopharmaceutical field due to their unique optical and surface plasmon resonance properties, straightforward design attributes and high degree of stability. They serve as effective chemical sensors and drug delivery agents and have been widely used in the cancer therapeutics and bio-imaging.
Preet and colleagues synthesised gold nanoparticles that were loaded with doxorubicin (DOX) and nisin (NIS) for treatment against murine skin cancer. The optimised plain gold nanoparticles (GNPs) were found to have a reported diametric value of 8–12 nm. The tumour volume was found to decrease significantly with doxorubicin-loaded nisin GNP treatment, demonstrating the therapeutic benefit of GNPs. [57]
Apigenin-linked gold nanoparticles (ap-AuNPs) have been reported to exhibit anticancer activity in epidermoid squamous carcinoma cells A431 via multiple mechanisms such as apoptosis and inhibition of angiogenesis. [58]
Ethosomes
Ethosomes are novel lipid vesicular agents containing relatively high concentrations of ethanol and phospholipids. The presence of alcohols incorporated in soft malleable vesicles ensures greater penetration in deeper lipidic dermal layers while imparting fluidic properties and membrane permeability. Due to their structural morphology, ethosomes can be used as carriers for hydrophilic as well as lipophilic high molecular weight molecules. Ethosomal administration has been reported to affect the lipidic stature of the stratum corneum. [59] Numerous studies have supported conclusions about improved entrapment effectiveness, permeation, and drug-deposition by ethosomes.
Binary ethosomes of fisetin were developed by Moolakkadath et al. for precise targeting of skin carcinogenic sites through dermal action. Confocal microscopy assessments demonstrated deeper penetration of the rhodamine B loaded ethosomes along with a concurrent two-fold greater reduction in the number of tumours on mice treated with binary ethosome gel in comparison to mice treated with UV therapy. This clearly indicates the tumour reduction ability of the gel. [60]
Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are spherical particles with a central core made of easily liquefiable solid lipids surrounded by an aqueous surfactant layer. SLNs are used as carriers for a variety of solid-lipid molecules including fatty acids, triglycerides, waxes, steroids, lipophilic drugs, and vaccines. SLNs offer the benefits of lower toxic effects, sustained drug release profiles, better cellular uptake, and higher bioavailability of the drugs. The distinctive quality of SLNs is to effectively target the epidermal layer to increase the benefit-risk ratio when therapeutic agents are applied topically. The lipidic environment of SLN provides protection against biological hindrance, reducing toxic episodes after targeted drug delivery.
SLN systems containing nonionic surfactants, curcumin (Cur) and resveratrol (Res) were developed by Palliyage et al. for topical treatment of MSC. Some of the findings of the study were better activity and stability of the Cur-Res SLNs in addition to a five-fold higher in-vitro release as well as permeability of resveratrol than curcumin. [61]
Silica nanoparticles
Mesoporous silica nanoparticles (MSNs) form honeycomb-structured nano materials, made up of a large number of two-dimensional mesopores. Structural parameters such as size, shape and surface area as well as treatment variables are crucial for their cytotoxicity. [62] They have tunable mesoporous structures with large pore volumes, which couled to their ease of surface modeling makes them useful multifunctional agents. [63] MSNs have been investigated as vehicles for gene transfection and hydrophobic medications for in vitro targeting, therapy, and imaging. [64]
Clemente et al. developed amine-functionalized MSNs verteporfin and investigated them against melanoma. In vitro experiments using red light-stimulated B16F10 mouse melanoma cells showed significant reduction in cell proliferation. The formulation also showed mitigatory effect on angiogenesis, lymphangiogenesis, and lung metastasis. [65]
Marinheiro et al. tested mesoporous silica nanoparticles of resveratrol against melanoma, factoring in various additional parameters such as pH-dependence and impact of amorphization. Resveratrol was found to crystallise inside the mesoporous matrix to their large pore sizes. The formulation demonstrated pH-mediated solubility and drug release with higher rates shown in the acidic tumour environment as compared to the physiological pH of 7.4. [66]
Nanofibers
Nanofibers (NFs) are fascinating solid fibres that have high porosity, surface-to-volume ratios and submicrometric diameter values. Natural as well as synthetic polymers such as chitosan, gelatin, hyaluronic acid, fibronectin, collagen, PLA, PLGA, PGA, PVP, PVA are used to create nanofibers for the topical delivery of medications. [67]
Balashanmugam et.al integrated polyvinyl alcohol-loaded gold nanoparticles and poly capro lactone-linked curcumin into a nanofiber-based platform of and demonstrated an induced apoptotic response and major reduction in the viability of A431 cells. [68]
Rengifo et al. developed carboxymethyl-hexanoyl chitosan and dodecyl sulphate nano-capsules loaded with pyrazoline based compounds with promising cytotoxicity against K-562 acute myeloid leukemia. [69]
Zhu et al. created dual-layered nanofibers with a 5-fluorouracil (5FU)-linked polyvinyl pyrollidine cores and chitosan-loaded poly-caprolactone shells and explored their synergistic potential against melanoma B16F10 cell lines. The observations demonstrated that the higher loading of chitosan enhanced drug loading, encapsulation efficiency and caused prominent aqueous diffusion through the fibres. The inhibitory response to B16F10 cells demonstrated a burst effect in the release profile of 5-FU, whereas the release of chitosan was sustained, thereby enhancing the cytotoxic effect, while lowering the potential for adverse events on normal cells. [70]
Conclusion
Numerous nanotech-based methods have been developed to bypass the drawbacks and shortcomings of traditional dermal therapy. Lipid-based nanocarriers, vesicular nanoparticles, polymeric nanoparticles, hydrogels and nanoemulsions are widely used sue to several favorable attributes such as targeted delivery, controlled release, better skin permeation, and enhanced stability, ultimately leading to enhanced drug efficacy, reduced side effects, and improved patient comfort and compliance. These techniques have higher efficacy, precision and better control of dosage delivery, target specificity, prolonged residence times and lesser side effects.
Lipid-based nanocarriers have the properties of good adherence to the skin's surface leading to better interaction with the stratum corneum. This close interaction results in higher amounts of drugs permeating the skin, thereby improving efficacy. Certain lipid-based nanocarriers, such as NLCs, have an amorphous structure that allows for greater drug retention and reduces the chances of drug loss during storage, leading to improved stability and efficacy. Upon topical application, lipid-based nanocarriers form an occlusive layer on the skin, which helps prevent water evaporation and increases skin moisture. This occlusive effect enhances drug permeation by maintaining hydration and facilitating drug diffusion through the skin layers. The higher penetrability and skin retention provided by these nanocarriers results in controlled release of drugs for extended period. This controlled release mechanism enhances drug efficacy and reduces the frequency of application. Nanoemulsions are hypothesized to extract and swell dermal lipids of damaged skin, resulting in higher bioavailability of included drugs. This mechanism facilitates the penetration of drugs into the irregular plaques characteristic of diseases like psoriasis.
Vesicular systems such as ethosomes, niosomes and transferosomes, exhibit excellent elasticity and deformability, reducing the likelihood of rupture and drug leakage during topical application. Ethosomes, containing high amounts of ethanol, disrupt the skin lipid barrier, facilitating deep skin penetration of drugs. Vesicles, such as niosomes, create a concentration gradient at the interface by adherance and fusion that serves as a catalyst for drug permeation across the stratum corneum. Additionally, vesicles possess permeation-enhancing qualities that facilitate drug transfer through the skin.
Silica-based materials are widely used for biomedical applications such as dermal drug delivery due to their biocompatibility and low toxicity potential. Mesoporous silica nanoparticles and nanofibers are promising platforms for dermal drug delivery possessing nanoscale dimensions, high surface areas and a network of interconnected pores, providing ample space for drug loading and encapsulation. This high porosity allows for high drug loading capacities and efficient drug release kinetics. Moreover, their pore size and surface can be precisely engineered, allowing for better control of drug release rates and skin interactions. This tunability enables customization of drug delivery profiles based on specific therapeutic requirements. Additionally, they shield encapsulated drugs from degradation and enzymatic activity in the skin microenvironment. This protection ensures the stability and efficacy of the delivered drugs.
The small-scale dimensions of nanoparticulate systems facilitate deep penetration into skin, enhancing drug permeation, bioavailability and delivery drugs to the skin or underlying tissues. The release kinetics of these nano-delivery systems can be tailored by adjusting factors such as pore size, surface functionalization, and external stimuli, providing precise control over drug release rates. Nanocarrier-based drug delivery has revolutionized the treatment of various skin disorders and represent a paradigm shift towards more effective, precise, and patient-friendly treatment strategies for various dermatological conditions.
Source of Funding
None.
Conflict of Interest
The authors declare that they have no conflict of interest. In addition, the authors declare that the work was done in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kaushik S, Lebwohl M. Psoriasis: Which therapy for which patient: Psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27-40. [Google Scholar]
- Murphy E, Schaffter S, Friedman A. Nanotechnology for Psoriasis Therapy. Curr Dermatol Rep. 2019;8:14-25. [Google Scholar] [Crossref]
- Palmer B, Delouise L. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules. 2016;21(12). [Google Scholar] [Crossref]
- Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci. 2019;20(6). [Google Scholar] [Crossref]
- Mao K, Fan Z, Yuan J, PC, Yang J, Xu J. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf B Biointerfaces. 2017;160:704-14. [Google Scholar] [Crossref]
- Badıllı U, Şen T, Tarımcı N. Microparticulate based topical delivery system of clobetasol propionate. AAPS PharmSciTech. 2011;12(3):949-57. [Google Scholar]
- Pradhan M, Singh D, Singh M. Influence of selected variables on fabrication of Triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif Cells Nanomed Biotechnol. 2016;44(1):392-400. [Google Scholar]
- Wang R. FK506-loaded solid lipid nanoparticles: Preparation, characterization and in vitro transdermal drug delivery. African J Pharm Pharmacol. 2012;6. [Google Scholar] [Crossref]
- Sathe P, Saka R, Kommineni N, Raza K, Khan W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev Ind Pharm. 2019;45(5):826-38. [Google Scholar]
- Gupta V, Kowshik K, Sehgal N. Development of nano structured lipid carrier based hydrogel for the treatment of psoriasis. Int J Res Pharm Sci. 2019;10(3):1711-9. [Google Scholar]
- Pinto M, Moura C, Nunes C, Segundo M, Lima S, Reis S. A new topical formulation for psoriasis: development of methotrexate-loaded nanostructured lipid carriers. Int J Pharm. 2014;477(1-2):519-26. [Google Scholar]
- Nam S, Ji X, Park J. Investigation of Tacrolimus Loaded Nanostructured Lipid Carriers for Topical Drug Delivery. Bull Korean Chem Soc. 2011;32:956-60. [Google Scholar] [Crossref]
- Lin Y, Huang Z, Zhuo R, Fang J. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int J Nanomedicine. 2010;5:117-28. [Google Scholar] [Crossref]
- Walunj M, Doppalapudi S, Bulbake U, Khan W. Preparation, characterization, and in vivo evaluation of cyclosporine cationic liposomes for the treatment of psoriasis. J Liposome Res. 2020;30(1):68-79. [Google Scholar]
- Lai R, Yeo R, Zhang B, Koh M, Tan S, Sim W. Clinical & Experimental Dermatology and Therapies Topical Application of Yolk Lecithin Liposomes Reinforces Skin Barrier Function Against Chemical Agents Such as Psoriasis- inducing IMQ and Alleviates Disease Phenotype. Clin Exp Dermatology Ther. 2018. [Google Scholar] [Crossref]
- Kumar R, Dogra S, Amarji B, Singh B, Kumar S, Sharma. Efficacy of Novel Topical Liposomal Formulation of Cyclosporine in Mild to Moderate Stable Plaque Psoriasis. JAMA Dermatol. 2016;152(7):807-15. [Google Scholar]
- Alam M, Ali M, Alam N, Alam M, Anwer T, Imam F. Design and Characterization of Nanostructure Topical Gel of Betamethasone Dipropionate for Psoriasis. J Appl Pharm Sci. 2012;2(10):148-58. [Google Scholar]
- Rajitha P, Shammika P, Aiswarya S, Gopikrishnan A, Jayakumar R, Sabitha M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J Drug Deliv Sci Technol. 2019;49:463-76. [Google Scholar] [Crossref]
- Sahu S, Katiyar S, Kushwah V, Jain S. Active natural oil-based nanoemulsion containing tacrolimus for synergistic antipsoriatic efficacy. Nanomedicine (Lond). 2018;13(16):1985-98. [Google Scholar]
- Musa S, Basri M, Masoumi HF, Shamsudin N, Salim N. Enhancement of physicochemical properties of nanocolloidal carrier loaded with cyclosporine for topical treatment of psoriasis: in vitro diffusion and in vivo hydrating action. Int J Nanomedicine. 2017;12:2427-41. [Google Scholar] [Crossref]
- Alam MS, Ali M, Zakir F, Alam N, Alam MI, Ahmad F. Enhancement of Anti-Dermatitis Potential of Clobetasol Propionate by DHA [Docosahexaenoic Acid] Rich Algal Oil Nanoemulsion Gel. Iran J Pharm Res. 2016;15(1):35-52. [Google Scholar]
- Duangjit S, Obata Y, Sano H, Kikuchi S, Onuki Y, Opanasopit P. Novel Ultradeformable Vesicles for Transdermal Drug Delivery: Optimization and Characterization. Biol Pharm Bull. 2012;35(10):1720-8. [Google Scholar]
- Morilla M, Romero E. Deformability in Drug Delivery. Curr Pharm Des. 2016;22(9):1-16. [Google Scholar] [Crossref]
- Srisuk P, Thongnopnua P, Raktanonchai U, Kanokpanont S. Physico-chemical characteristics of methotrexate-entrapped oleic acid-containing deformable liposomes for in vitro transepidermal delivery targeting psoriasis treatment. Int J Pharm. 2012;427(2):426-34. [Google Scholar]
- Trotta M, Peira E, Carlotti ME, Gallarate M. Deformable liposomes for dermal administration of methotrexate. Int J Pharm. 2004;270(1-2):119-25. [Google Scholar]
- Gizaway S, Fadel M, Mourad B, Elnaby F. Betamethasone Dipropionate Gel For Treatment Of Localized Plaque Psoriasis. Int J Pharm Pharm Sci. 2017;9(8):173-82. [Google Scholar]
- Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int J Pharm. 2018;535(1-2):1-17. [Google Scholar]
- Lei W, Yu C, Lin H, Zhou X. Development of tacrolimus-loaded transfersomes for deeper skin penetration enhancement and therapeutic effect improvement in vivo. Asian J Pharm Sci. 2013;8(6):336-45. [Google Scholar]
- Cevc G, Blume G. Hydrocortisone and dexamethasone in very deformable drug carriers have increased biological potency, prolonged effect, and reduced therapeutic dosage. Biochim Biophys Acta. 2004;1663(1-2):61-73. [Google Scholar]
- Sudhakar C, Jain S, Charyuliu R. Comparison Study of Liposomes, Transfersomes and Ethosomes Bearing Lamivudine. Int J Pharm Sci Res. 2016;7(10):4214-21. [Google Scholar]
- Zhang Y, Xia Q, Li Y, He Z, Li Z, Guo T. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics. 2019;9(1):48-64. [Google Scholar]
- Zhang Y, Feng N, Shen L, Zhao J. Evaluation of psoralen ethosomes for topical delivery in rats by using in vivo microdialysis. Int J Nanomedicine. 2014;9:669-78. [Google Scholar]
- Li G, Fan Y, Fan C, Li X, Wang X, Li M. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm. 2012;82(1):49-57. [Google Scholar]
- Garg T, Rath G, Goyal A. Nanotechnological approaches for the effective management of psoriasis. Artif Cells Nanomed Biotechnol. 2016;44(6):1374-82. [Google Scholar]
- Hashim I, El-Magd N, El-Sheakh A, Hamed M, AAE. Pivotal role of Acitretin nanovesicular gel for effective treatment of psoriasis: ex vivo–in vivo evaluation study. Int J Nanomedicine. 2018;13:1059-79. [Google Scholar] [Crossref]
- Priprem A, Damrongrungruang T, Limsitthichaikoon S, Khampaenjiraroch B, Nukulkit C, Thapphasaraphong S. Topical Niosome Gel Containing an Anthocyanin Complex: a Potential Oral Wound Healing in Rats. AAPS PharmSciTech. 2018;19(4):1681-92. [Google Scholar]
- Abdelbary AA, Aboughaly M. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box-Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int J Pharm. 2015;485(1-2):235-43. [Google Scholar]
- Johnson B, Franco A, Beck L, Prezzano J. Treatment-resistant atopic dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:181-92. [Google Scholar] [Crossref]
- Hussain Z, Katas H, Amin M, Kumolosasi E, Buang F, Sahudin S. Self-assembled polymeric nanoparticles for percutaneous co-delivery of hydrocortisone/hydroxytyrosol: an ex vivo and in vivo study using an NC/Nga mouse model. Int J Pharm. 2013;444(1-2):109-19. [Google Scholar]
- Rosado C, Silva C, Reis C. Hydrocortisone-loaded poly(ε-caprolactone) nanoparticles for atopic dermatitis treatment. Pharm Dev Technol. 2013;18(3):710-8. [Google Scholar]
- Siddique M, Katas H, Jamil A, MA, Ng S, Zulfakar M. Potential treatment of atopic dermatitis: tolerability and safety of cream containing nanoparticles loaded with hydrocortisone and hydroxytyrosol in human subjects. Drug Deliv Transl Res. 2019;9(2):469-81. [Google Scholar]
- Badihi A, Frušić-Zlotkin M, Soroka Y, Benhamron S, Tzur T, Nassar T. Topical nano-encapsulated cyclosporine formulation for atopic dermatitis treatment. Nanomedicine. 2020;24. [Google Scholar] [Crossref]
- Kang J, Chon J, Kim Y, Lee H, Oh D, Lee H. Preparation and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution. Int J Nanomedicine. 2019;14:5381-96. [Google Scholar] [Crossref]
- Ilves M, Palomäki J, Vippola M, Lehto M, Savolainen K, Savinko T. Topically applied ZnO nanoparticles suppress allergen induced skin inflammation but induce vigorous IgE production in the atopic dermatitis mouse model. Part Fibre Toxicol. 2014;11. [Google Scholar] [Crossref]
- Yanagisawa R, Takano H, Inoue K, Koike E, Sadakane K, Ichinose T. Size effects of polystyrene nanoparticles on atopic dermatitislike skin lesions in NC/NGA mice. Int J Immunopathol Pharmacol. 2010;23(1):131-41. [Google Scholar]
- Kang H, Kim S, Lee K, Jin S, Kim S, Jeon H. 5 nm Silver Nanoparticles Amplify Clinical Features of Atopic Dermatitis in Mice by Activating Mast Cells. Small. 2017;13(9). [Google Scholar] [Crossref]
- Akhtar N, Verma A, Pathak K. Investigating the penetrating potential of nanocomposite β-cycloethosomes: development using central composite design, in vitro and ex vivo characterization. J Liposome Res. 2018;28(1):35-48. [Google Scholar]
- Carreras J, Tapia-Ramirez W, Sala A, Guillot A, Garrigues T, Melero A. Ultraflexible lipid vesicles allow topical absorption of cyclosporin A. Drug Deliv Transl Res. 2020;10(2):486-97. [Google Scholar]
- Baspinar Y, Borchert H. Penetration and release studies of positively and negatively charged nanoemulsions-Is there a benefit of the positive charge. Int J Pharm. 2012;430(1-2):247-52. [Google Scholar]
- Espinoza L, Silva-Abreu M, Calpena A, Rodríguez-Lagunas M, Fábrega M, Garduño-Ramírez M. Nanoemulsion strategy of pioglitazone for the treatment of skin inflammatory diseases. Nanomedicine. 2019;19:115-25. [Google Scholar] [Crossref]
- Croisfelt F, Tundisi L, Ataide J, Silveira E, Tambourgi E, Jozala A. Modified-release topical hydrogels: a ten-year review. J Mater Sci. 2019;54:10963-83. [Google Scholar] [Crossref]
- Lee H, Kim T, Oh S, Lee J. Prednisolone-loaded coatable polyvinyl alcohol/alginate hydrogel for the treatment of atopic dermatitis. J Biomater Sci Polym Ed. 2018;29(13):1612-24. [Google Scholar]
- Napimoga M, Clemente-Napimoga J, Machabanski N, Juliani M, Acras P, Macedo C. The 15d-PGJ2 hydrogel ameliorates atopic dermatitis through suppression of the immune response. Mol Med Rep. 2019;19(6):4536-44. [Google Scholar]
- Eroğlu �, Azizoğlu E, Özyazıcı M, Nenni M, HO, Özbal S. Effective topical delivery systems for corticosteroids: dermatological and histological evaluations. Drug Deliv. 2016;23(5):1502-13. [Google Scholar]
- Calienni MN, Febres-Molina C, Llovera R, Zevallos-Delgado C, Tuttolomondo M, Paolino D. Nanoformulation for potential topical delivery of Vismodegib in skin cancer treatment. Int J Pharm. 2019;565:108-22. [Google Scholar] [Crossref]
- Caddeo C, Nacher A, Vassallo A, Armentano MF, Pons R, Fernàndez-Busquets X. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int J Pharm. 2016;513(1-2):153-63. [Google Scholar]
- Preet S, Pandey S, Kaur K, Chauhan S, Saini A. Gold nanoparticles assisted co-delivery of nisin and doxorubicin against murine skin cancer. J Drug Deliv Sci Technol. 2019;53. [Google Scholar] [Crossref]
- Rajendran I, Dhandapani H, Anantanarayanan R, Rajaram R. Apigenin mediated gold nanoparticle synthesis and their anti-cancer effect on human epidermoid carcinoma (A431) cells. RSC Adv. 2015;5:51055-66. [Google Scholar] [Crossref]
- Niu X, Zhang D, Bian Q, Feng X, Li H, Rao Y. Mechanism investigation of ethosomes transdermal permeation. Int J Pharm X. 2019;1. [Google Scholar] [Crossref]
- Moolakkadath T, Aqil M, Ahad A, Imam S, Praveen A, Sultana Y. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int J Pharm. 2019;560:78-91. [Google Scholar] [Crossref]
- Palliyage GH, Hussein N, Mimlitz M, Weeder C, Alnasser M, Singh S. Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm Res. 2021;38(5):851-71. [Google Scholar]
- Vivero-Escoto J, Slowing I, Trewyn B, Lin V. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small. 2010;6(18):1952-67. [Google Scholar]
- Wu K, Yamauchi Y. Controlling physical features of mesoporous silicananoparticles (MSNs) for emerging applications. J Mater Chem. 2012;22:1251-6. [Google Scholar] [Crossref]
- Cholkar K, Hirani N, Natarajan C. Nanotechnology-Based Medical and Biomedical Imaging for Diagnostics. Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices. 2017. [Google Scholar] [Crossref]
- Clemente N, Miletto I, Gianotti E, Invernizzi M, Marchese L, Dianzani U. Verteporfin-loaded mesoporous silica nanoparticles inhibit mouse melanoma proliferation in vitro and in vivo. J Photochem Photobiol B. 2019;197. [Google Scholar] [Crossref]
- Marinheiro D, Ferreira B, Oskoei P, Oliveira H, Daniel-Da-Silva A. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials (Basel). 2021;14(6). [Google Scholar] [Crossref]
- Goyal R, Macri L, Kaplan H, Kohn J. Nanoparticles and nanofibers for topical drug delivery. J Control Release. 2016;240:77-92. [Google Scholar] [Crossref]
- Balashanmugam P, Sucharithra G, Mary S, Selvi A. Efficacy of biopolymeric PVA-AuNPs and PCL-Curcumin loaded electrospun nanofibers and their anticancer activity against A431 skin cancer cell line. Mater Today Commun. 2020;25. [Google Scholar] [Crossref]
- AR, Stefanes N, Toigo J, CM, MS, Nunes R. A new and efficient carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanocarrier for a pyrazoline with antileukemic activity. Mater Sci Eng C Mater Biol Appl. 2019;105. [Google Scholar] [Crossref]
- Zhu L, Zheng Y, Fan J, Yao Y, Ahmad Z, Chang M. A novel core-shell nanofiber drug delivery system intended for the synergistic treatment of melanoma. Eur J Pharm Sci. 2019;137. [Google Scholar] [Crossref]
- Abstract
- Introduction
- Psoriasis
- Polymeric nanoparticles
- Solid lipid nanoparticles (SLN)
- Nanostructured lipid carriers (NLC
- Liposomes
- Nanoemulsions
- Deformable liposomes
- Transferosomes
- Ethosomes
- Niosomes
- Atopic Dermatitis (AD)
- Polymeric nanoparticles
- Inorganic nanocarriers
- Lipid-based nanocarriers
- Liposomes
- Nanoemulsions
- Hydrogels systems
- Skin Cancers
- Conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Sharma P, Prabhu A. Nanocarrier-Mediated dermal drug delivery for skin disorders [Internet]. IP Indian J Clin Exp Dermatol. 2025 [cited 2025 Oct 24];11(1):10-21. Available from: https://doi.org/10.18231/j.ijced.2025.002
APA
Sharma, P., Prabhu, A. (2025). Nanocarrier-Mediated dermal drug delivery for skin disorders. IP Indian J Clin Exp Dermatol, 11(1), 10-21. https://doi.org/10.18231/j.ijced.2025.002
MLA
Sharma, Priya, Prabhu, Arati. "Nanocarrier-Mediated dermal drug delivery for skin disorders." IP Indian J Clin Exp Dermatol, vol. 11, no. 1, 2025, pp. 10-21. https://doi.org/10.18231/j.ijced.2025.002
Chicago
Sharma, P., Prabhu, A.. "Nanocarrier-Mediated dermal drug delivery for skin disorders." IP Indian J Clin Exp Dermatol 11, no. 1 (2025): 10-21. https://doi.org/10.18231/j.ijced.2025.002
