- Visibility 1.2k Views
- Downloads 56 Downloads
- Permissions
- DOI 10.18231/j.ijced.2020.036
-
CrossMark
- Citation
Correlation of levels of serum testosterone and dhea-s in patients of acne vulgaris
- Author Details:
-
Farahnaaz Hashmi
-
Syed Yousuf Ali *
-
Shaik Noor Fazal
-
Uzma Fatima
Abstrac
Background: Acne is one of the most commonest dermatological disorders in young patients. 1. It is a disease of the pilosebaceous follicular unit. 2. Endocrine mechanisms control the components of sebocyte function—namely lipid synthesis, proliferation and differentiation. 3. Androgens upregulate the sebaceous glandular function by binding to the nuclear androgen receptors (ARs). Highest density of these androgen receptors have been demonstrated in sebaceous glands.
The mechanisms suggested in acne include: Increased circulating levels of androgens, Increased free androgen levels, Increased local metabolism of androgens in skin and increased tissue sensitivity to androgens.
Materials and Methods: This case control study was conducted In the department of dermatology, venerology and leprosy, shadan institute of medical sciences, Hyderabad from February 2019-february-2020. A total of 50 patients (25 males and 25 females) of moderate to severe acne vulgaris resistant to conventional treatment were included in the study A total of 50 patients (25 males and 25 females) of moderate to severe acne vulgaris resistant to conventional treatment were included in the study
Inclusion criteria: Age range: 14 to 35 years
Exclusion criteria: patients who were receiving any systemic drugs, oral contraceptives or hormonal therapy, pregnant and lactating. 50 age and sex matched healthy acne free subjects were also part of the study.
Methods: Venous blood samples were collected for the assay of serum testosterone, dehydroepiandrosterone-sulfate (DHEA-S) and androstenedione (A4A) between 0800-0900 hours. For testosterone assay three samples were collected at 20 minute intervals and pooled together. Immediately after collection the red cells were separated and the sera were stored at - 20°C till assay. In females, the samples were collected only in the first half of the follicular phase of the menstrual cycle. All hormones were measured by radioimmunoassay using standard techniques with commercially obtained kits.
Result: All patients included in the study had moderate to severe acne. The commonest lesions encountered were pustules and nodules while cysts were present in 10/25 males and 11/25 females. 32/50 patients had acne lesions also on the trunk. The mean duration of acne was 2.6 years (range 1 to 6 years). A history of partial or no response to conventional treatment was present in all the patients. Mild to moderate degree of hirsutism was present in 7/25 female patients; of whom 4 also had oligomenorrhea. No other sign of virilisation was observed in any female patient. In males with acne the mean serum testosterone levels were only marginally elevated but their DHEA-S levels were significantly higher than in controls.
Introduction
Acne vulgaris is a commonest disorder of the pilosebaceous unit. Its mean prevalence in adolescence is estimated to be 70-87%.[1] Its cutaneous manifestation is well known to clinicians and have been amply described. The endocrine causes and associated disease states are less commonly described. Hence, the hormonal pathogenesis of acne is well emphasized in this article.
Sebaceous glands and sebum production play a central role in the development of acne. Sebaceous gland is hormonally regulated which in turn affects the pilosebaceous unit.
Androgens
Perhaps the most well known effect of hormones on the pilosebaceous unit is the one caused by androgens, more specifically:
Sebaceous gland enlargement
Sebocyte proliferation
Majority of the circulating androgens are produced by gonads and the adrenal gland, but they are also locally produced, in sebocyte from dehydroepiandrosterone (DHEA) sulfate, an adrenal precursor hormone.
Mechanism
Androgen receptors are expressed in the basal layer of the sebaceous gland and in the outer root sheath keratinocytes of the hair follicle.[4], [5]
When free testosterone enters the cell, It is quickly reduced to 5-dihydrotestosterone (5-DHT) by the 5α-reductase enzyme.
The activity of 5α-reductase is increased in the sebaceous gland in proportion to the size of the gland.[6]
DHT is ~5-10 times more potent than testosterone in its interaction with the androgen receptor.
On binding to its receptor protein, DHT is translocated to the nucleus and initiates the transcription of androgen responsive genes.
DHT increases the mRNA of proteins involved in fatty acid, triglyceride squalene, and cholesterol synthesis.
This effect is mediated by sterol response element binding proteins (SREBP’s
By inhibiting SREBP’s effect with 25-hydroxy cholesterol, there was a 50% decrease in lipid synthesis increase by DHT alone. [7]
Androgens exert their effect on sebaceous glands by increasing the proliferation of sebocytes and increasing lipid production through SREBP’s.[8]
It is now clear that pilosebaceous units possess all the steroid metabolizing enzymes needed to convert DHEA sulfate (DHEAS) to the mid potent androgen, DHT including 3β-hydroxysteroid dehydrogenase [9] and 5α-reductase.[10]
Type-1 isoenzyme of 5α-reductase and Type-2 isoenzyme of 17β-hydroxysteroid dehydrogenase are predominant in sebaceous gland,[11], [12] infundibular keratinocytes, and epidermis.
Furthermore, sebocytes have the biosynthetic capacity to produce their own androgen from cholesterol through the Cytp450 side-chain cleavage system (P450 Scc).[13]
Along with its cofactors adenodoxin, adrenodoxin reductase, and the transcription factor, steroidogenic Factor 1, P450 scc converts cholesterol to pregnenolone, which is also the precursor for estrogen synthesis.
Conclusion from this theory is that the skin has its own capacity to metabolize androgens suggesting that skin exercises local control over the ultimate effects of circulating androgen on the target tissue.
The mechanisms suggested include
Increased circulating levels of androgens
Increased free androgen levels16
Increased local metabolism of androgens in skin and
Increased tissue sensitivity to androgens.
As there is inadequate evidence on the status of circulating androgens and severity of acne in patients, this study was undertaken to evaluate the same.
Materials and Methods
This case control study was conducted In the Department of Dermatology, Venerology and Leprosy, Shadan Institute of Medical Sciences, Hyderabad from February 2019-February-2020.
A total of 50 patients (25 males and 25 females) of moderate to severe acne vulgaris resistant to conventional treatment were included in the study
Inclusion criteria
Age range : 14 to 35 years
Exclusion criteria
Patients receiving any systemic drugs
Patients reiceiving oral contraceptives
Patients receiving hormonal therapies
Patients who are immunocompromised
Patients who are pregnant and lactating
Methods
Venous blood samples were collected for the assay of serum testosterone, dehydroepiandrosterone-sulfate (DHEA-S) and androstenedione (A4A) between 0800-0900 hours.
For testosterone assay three samples were collected at 20 minute intervals and pooled together.
Immediately after collection the red cells were separated and the sera were stored at - 20°C till assay.
In females, the samples were collected only in the first half of the follicular phase of the menstrual cycle.
All hormones were measured by radioimmunoassay using standard techniques with commercially obtained kits.
Results
All patients included in the study had moderate to severe acne. The commonest lesions encountered were pustules and nodules while cysts were present in 10/25 males and 11/25 females.
32/50 patients had acne lesions also on the trunk.
The mean duration of acne was 2.6 years (range 1 to 6 years).
A history of partial or no response to conventional treatment was present in all the patients.
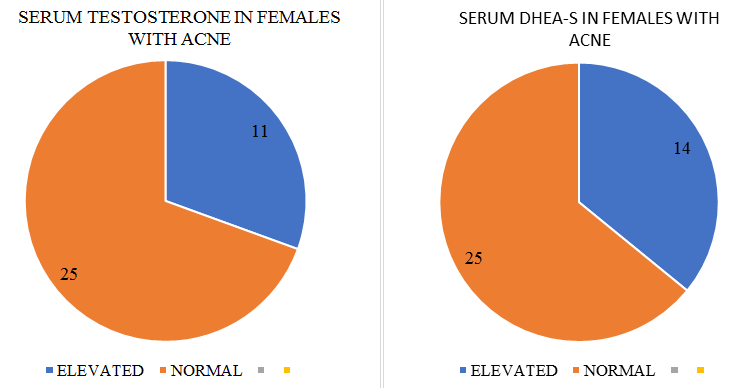
Mild to moderate degree of hirsutism was present in 7/25 female patients; of whom 4 also had oligomenorrhea.
No other sign of virilisation was observed in any female patient.
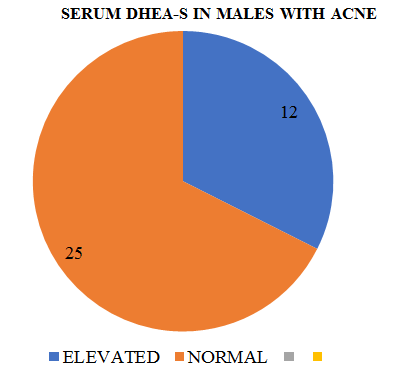
In males with acne the mean serum testosterone(17) levels were only marginally elevated but in 12/25 DHEA-S levels were significantly higher in controls.
.
Discussion
The status of basal serum androgens in patients of acne vulgaris remains controversial. Certain previous studies have reported raised serum testosterone and DHEA-S levels in females with acne while others have found only free testosterone to be raised.[14]
It has earlier been observed that serum testosterone levels in males with acne are similar to those in controls. We found only marginally higher levels of serum testosterone in our male patients.
Since DHEA-S almost wholly and A4A is partly secreted from the adrenal cortex and can be peripherally converted to testosterone[15] and dihydrotestosterone, it is possible that there may be a state of adrenal hypersecretion in patients with recalcitrant acne.
This may, however, not be severe enough to produce other features of hyperandrogenism.
Acne lesions are known to aggravate with emotional stress, perhaps by way of an overactive pituitary adrenal axis.
These patients should be investigated to exclude adrenal enzyme defects as treatment with anti-androgens is likely to be rewarding in such type of patients.
Conclusion
Although androgenic hormones may be raised in the acne patients as compared to controls, only Testosterone and DHEA-S levels serve as markers of acne severity.
Any therapy targeted towards acne and its severity can, therefore, be directed towards the ovarian androgen production, or blocking of androgen receptors in the pilosebaceous unit.
Thus hormonal therapy can be an adjuvant to early treatment in acne
Source of Funding
None.
Conflict of Interest
None.
References
- Dreno B, Poli F. Epidemiology of Acne. Dermatol. 2003;206(1):7-10. [Google Scholar]
- Hamilton JB. Male hormone substance: A prime factor in acne. J Clin Endocrinol . 1941;1(7):570-92. [Google Scholar]
- Rosenfield RL, Deplewski D. Role of androgens in the developmental biology of the pilosebaceous unit. Am J Med . 1995;98(1):S80-8. [Google Scholar]
- Choudhry R, Hodgins MB, Kwast THVd, Brinkmann AO, Boersma WJA. Localization of androgen receptors in human skin by immunohistochemistry: implications for the hormonal regulation of hair growth, sebaceous glands and sweat glands. J Endocrinol. 1992;133(3):467-75. [Google Scholar]
- Liang T, Hoyer S, Yu R, Soltani K, Lorincz AL, Hiipakka RA. Immunocytochemical Localization of Androgen Receptors in Human Skin Using Monoclonal Antibodies Against the Androgen Receptors. J Invest Dermatol . 1993;100(5):663-6. [Google Scholar]
- Dijkstra AC, Goos C, Cunliffe WJ, Sultan C, Vermorken AJ. Is Increased 5a-Reductase Activity a Primary Phenomenon in Androgen-Dependent Skin Disorders?. J Invest Dermatol . 1987;89(1):87-92. [Google Scholar]
- Rosignoli C, Nicolas JC, Jomard A, Michel S. Involvement of the SREBP pathway in the mode of action of androgens in sebaceous glands in vivo. Exp Dermatol. 2003;12(4):480-9. [Google Scholar]
- Lawrence DM, Katz M, Robinson T. Reduced sex hormone binding globulin and free testosterone levels in women with severe acne. J Clin Endocr. 1981;15:87-91. [Google Scholar]
- Sawaya ME, Penneys NS. Immunohistochemical distribution of aromatase and 3B-hydroxysteroid dehydrogenase in human hair follicle and sebaceous gland. Journal of Cutaneous Pathology. 1992;19(4):309-14. [Google Scholar]
- Thiboutot D, Martin P, Volikos L, Gilliland K. Oxidative Activity of the Type 2 Isozyme of 17β-Hydroxysteroid Dehydrogenase (17β-HSD) Predominates in Human Sebaceous Glands. J Invest Dermatol. 1998;111(3):390-5. [Google Scholar]
- Luu-The V, Sugimoto Y, Puy L, Labrie Y, Solache IL, Singh M. Characterization, Expression, and Immunohistochemical Localization of 5α-Reductase in Human Skin. J Invest Dermatol . 1994;102(2):221-6. [Google Scholar]
- Bayne E, Flanagan J, Einstein M, Ayala J, Chang B, Azzolina B, et al. Immunohistochemical localization of types 1 and 2 5α-reductase in human scalp. Br J Dermatol . 1999;141(3):481-91. [Google Scholar]
- Thiboutot DM, Knaggs H, Gilliland K, Hagari S. Activity of type 1 5 alphareductase is greater in the follicular infrainfundibulum compared with the epidermis. Br J Dermatol. 1997;136:166-71. [Google Scholar]
- Chen W, Thiboutot D, Zouboulis CC. Cutaneous Androgen Metabolism: Basic Research and Clinical Perspectives. J Invest Dermatol. 2002;119(5):992-1007. [Google Scholar]
- Takayasu S, Wakimoto H, Itami S, Sano S. Activity of Testosterone 5α-Reductase in Various Tissues of Human Skin. J Invest Dermatol. 1980;74(4):187-91. [Google Scholar]
How to Cite This Article
Vancouver
Hashmi F, Ali SY, Fazal SN, Fatima U. Correlation of levels of serum testosterone and dhea-s in patients of acne vulgaris [Internet]. IP Indian J Clin Exp Dermatol. 2020 [cited 2025 Oct 19];6(2):183-186. Available from: https://doi.org/10.18231/j.ijced.2020.036
APA
Hashmi, F., Ali, S. Y., Fazal, S. N., Fatima, U. (2020). Correlation of levels of serum testosterone and dhea-s in patients of acne vulgaris. IP Indian J Clin Exp Dermatol, 6(2), 183-186. https://doi.org/10.18231/j.ijced.2020.036
MLA
Hashmi, Farahnaaz, Ali, Syed Yousuf, Fazal, Shaik Noor, Fatima, Uzma. "Correlation of levels of serum testosterone and dhea-s in patients of acne vulgaris." IP Indian J Clin Exp Dermatol, vol. 6, no. 2, 2020, pp. 183-186. https://doi.org/10.18231/j.ijced.2020.036
Chicago
Hashmi, F., Ali, S. Y., Fazal, S. N., Fatima, U.. "Correlation of levels of serum testosterone and dhea-s in patients of acne vulgaris." IP Indian J Clin Exp Dermatol 6, no. 2 (2020): 183-186. https://doi.org/10.18231/j.ijced.2020.036
