- Visibility 141 Views
- Downloads 8 Downloads
- DOI 10.18231/j.ijced.2025.015
-
CrossMark
- Citation
A prospective, double-blind, placebo-controlled single-center, randomized controlled trial to evaluate the efficacy of a multi-strain probiotic on chronic spontaneous urticaria
- Author Details:
-
Kiran Vasant Godse
-
Atul Amritrao Deshmukh *
-
Ajinkya Amritrao Deshmukh
-
Vatsal Patidar
-
Vijayraghavan Seshadri
Introduction
Chronic urticaria, defined as wheals or angioedema lasting more than six weeks, is considered chronic when symptoms occur spontaneously and recur daily or almost daily over this period. It is termed "spontaneous" when no identifiable external triggers are present. Although not life-threatening, chronic urticaria can significantly impact a patient’s quality of life. The first-line treatment typically involves modern, second-generation H1-antihistamines. For patients unresponsive to these treatments, options include a short course of systemic corticosteroids, omalizumab, or ciclosporin. However, both ciclosporin and long-term steroid use are associated with toxicities and adverse side effects, underscoring the need for safer, more effective treatments for chronic spontaneous urticaria (CSU). [1], [2], [3], [4]
Probiotics have been extensively researched for their role in regulating gut health for over a century. One of their key functions is modulating the gut microbiota, which is closely linked to the intestinal immune system and a reduction in allergic responses. Recently, evidence suggests that some probiotic strains may also influence the skin’s immune response through the gut-skin axis. Clinical trials in humans have demonstrated the potential of probiotics in alleviating atopic dermatitis, and growing data indicate that changes in gut microflora composition or volume may affect IgE responses. [5], [6], [7]
Probiotics can reduce the secretion of pro-Th2 cytokines from THP-1 cells, promoting a healthier Th1/Th2 balance. Th2 cells play a crucial role in allergic reactions and the production of IgE antibodies. In the context of CSU, IgE antibodies, FcεRI receptors, and mast cells are believed to be important pathogenic factors, although the exact cause of the condition remains unidentified. [8], [9]
The primary goal of this study is to assess the clinical efficacy, safety, and impact on quality of life in CSU patients before and after adding a multi-strain probiotic (Lactogut) to their standard treatment regimen.
Study rationale
As noted, probiotics have demonstrated benefits in managing chronic skin conditions. Currently, corticosteroids are used to treat refractory and recurrent cases of CSU, but their long-term use is limited by significant side effects. There is a need for alternative therapies to minimize or avoid these adverse effects, and probiotics offer a promising option. This study aims to investigate the role of probiotics in treating CSU.
Objectives
The primary efficacy objective of the study was to evaluate multi-strain probiotic's ability to provide both clinical and subjective relief from chronic spontaneous urticaria (CSU) after 12 weeks of treatment. The secondary efficacy objectives focused on assessing changes in urticaria activity scores and urticaria control tests from baseline to the conclusion of the 12-week treatment period. Additionally, the exploratory efficacy objective aimed to compare the efficacy of multi-strain probiotic with conventional therapy alone in treating CSU. The safety objective was to monitor and evaluate any adverse events (AEs) occurring throughout the duration of the study.
Materials and Methods
Study design
This was a prospective, single-center, double-blind, placebo-controlled, investigator-initiated randomized controlled trial designed to assess the efficacy of multi-strain probiotic in treating patients with chronic spontaneous urticaria (CSU). The sample size for the study was determined using a 95% confidence interval and a 5% margin of error. Based on a literature review indicating that 98% of the population exhibits control over itch severity, the minimum required sample size for each group was calculated to be 40 participants.
All the participants attended four scheduled visits at the study site throughout the study. Visit 1, Screening and baseline assessment (within 7 days of enrollment), visit 2, Interim assessment at 4 weeks (± 4 days), visit 3, Interim assessment at 8 weeks (± 4 days) and visit 4, End-of-treatment (EOT) assessment at 12 weeks (± 4 days).
Trial was prospectively registered to the clinical trial registry of India with a CTRI number of CTRI/2023/06/054428. Ethical clearance for the study was obtained from the Institutional Ethics Committee (IEC Reference Number, IEC Ref. No.DYP/IEC/12/2023). The study was conducted in accordance with the principles outlined in the Declaration of Helsinki (2000) and adhered to good clinical practice guidelines. Patients were informed about the study procedures, and informed consent was obtained from those who were willing to participate.
Inclusion criteria included male and female volunteers aged 18 years or older, with a clinical history of CSU lasting more than six weeks and experiencing hives on more than three days per week. Participants were required to fully engage in the study and show a willingness to comply with all protocol procedures. Each participant had to demonstrate the ability to understand and provide voluntary, written informed consent for their participation. Additionally, subjects agreed not to make significant changes to their diet, medications, or exercise routines during the study.
Exclusion criteria included patients who had used probiotic supplements within the 4 weeks prior to screening. Additionally, individuals deemed uncooperative, pregnant or lactating women, and those with primary or acquired immunodeficiency, including HIV seropositivity, were not eligible. Subjects who were receiving or planning to receive investigational new drugs (IND), ultraviolet light therapy, monoclonal antibodies, or systemic immunosuppressants were also excluded. The use of topical or oral complementary and alternative medicine (CAM) agents within 4 weeks of treatment initiation disqualified participants, as did hypersensitivity to any components in the probiotic capsules or sachets. Cognitively impaired individuals or those unable to provide informed consent were not permitted to participate. Patients with significant concomitant illnesses, such as malignancies, psychiatric disorders, or major systemic diseases (e.g., hepatic or endocrine conditions), were also excluded. Finally, any other condition that, in the Investigator’s opinion, could affect a participant’s ability to complete the study or pose a significant risk to their safety resulted in exclusion.
Upon signing the informed consent form (ICF) during visit 1(baseline visit), patients underwent screening procedures and assessments. During this period, demographic information was collected, eligibility criteria were reviewed, and the diagnosis of chronic spontaneous urticaria (CSU) was confirmed. Basic laboratory tests, including a complete blood count (CBC), random blood sugar levels, and thyroid-stimulating hormone (TSH) levels, were conducted to ensure patient safety. Medical and surgical histories, along with details of prior and current medications, were also documented. The screening process lasted up to 7 days, during which patients could visit the study site multiple times if necessary. Physical examinations were performed, and vital signs were recorded. CSU was diagnosed using the urticaria activity score and the urticaria control test (UCT). After completing the screening, eligible patients were randomly assigned to either the experimental or control arm. The experiment arm received multi-strain probiotic in addition to conventional therapy, while the control group received conventional therapy with a placebo. Each multi-strain probiotic capsule (Lactogut) contains a proprietary blend of 5 billion CFU of the following probiotic strains: *Bacillus coagulans* unique IS-2, *Lactobacillus rhamnosus* UBLR-58, *Bifidobacterium longum* UBBL-64, *Bifidobacterium bifidum* UBBB-55, *Saccharomyces boulardii* unique-28, and *Streptococcus thermophilus* UBST-50, along with 20 mg of fructooligosaccharides and 10 mg of lactitol. Multi-strain probiotic was administered orally, with one capsule taken twice daily for 12 weeks, in conjunction with conventional therapy in a treatment group. Conventional therapy included levocetirizine, and patients who were intolerant to levocetirizine were given Bilastine as an alternative. A total of 97 patients with CSU were recruited and evenly distributed between the treatment and control groups. The study treatment began on Day 1. During visit 2, after 4 weeks of the treatment, and visit 3, after 8 weeks of the treatment, physical examination was conducted, and vital signs were recorded. All participants were evaluated using the urticaria activity score and the urticaria control test to assess the severity and control of their condition during visit 2 and 3. All end-of-treatment (EOT) assessments and procedures were conducted the day following the EOT visit (i.e., at 12 weeks). Participants underwent evaluation using the urticaria activity score and urticaria control test to assess their condition. All relevant prior medications taken within the last three months of screening were recorded, along with any medical and surgical history from the six months preceding screening, which was documented in the case report form (CRF). During the study, all medications administered to the patients were also recorded in the CRF. Any prior medications that patients continued during the study were noted in the source documents and in the concomitant section of the CRF. The information on concomitant medications included, but was not limited to, the generic name, indication, total daily dose (unit, frequency, route), start date/time, stop date/time, and whether the medication was ongoing. It is important to note that antiepileptics, other probiotics, and anti-cancer therapies were prohibited during the study. The primary efficacy endpoint of the study was the number of patients experiencing complete relief from chronic spontaneous urticaria (CSU) after 12 weeks of treatment. Secondary efficacy endpoints included the percentage change in urticaria activity scores and urticaria control tests at affected sites following multi-strain probiotic treatment after 12 weeks from baseline. Additionally, the safety endpoint assessed the safety of the investigational product by evaluating clinically significant changes in physical examination details, vital signs, and laboratory investigations throughout the study period. During each visit urticaria activity score (UAS) ([Table 1]), urticaria control test ([Table 2]) and adverse events were recorded.
A patient was free to withdraw consent from the study at any time. Additionally, a patient could be discontinued from the trial at any point if the Investigator deemed it necessary. In such cases, the reason for treatment discontinuation was recorded in the case report form (CRF). Patients who withdrew during the screening period were classified as screen failures. For patients who were withdrawn from treatment during the treatment period, the assessments and procedures from Visit 3 were completed. All patients who were identified as screen failures or who withdrew early from the study treatment during the treatment period were appropriately discontinued and received treatment for their medical condition according to the standard of care, as determined by the Investigator’s discretion.
Results
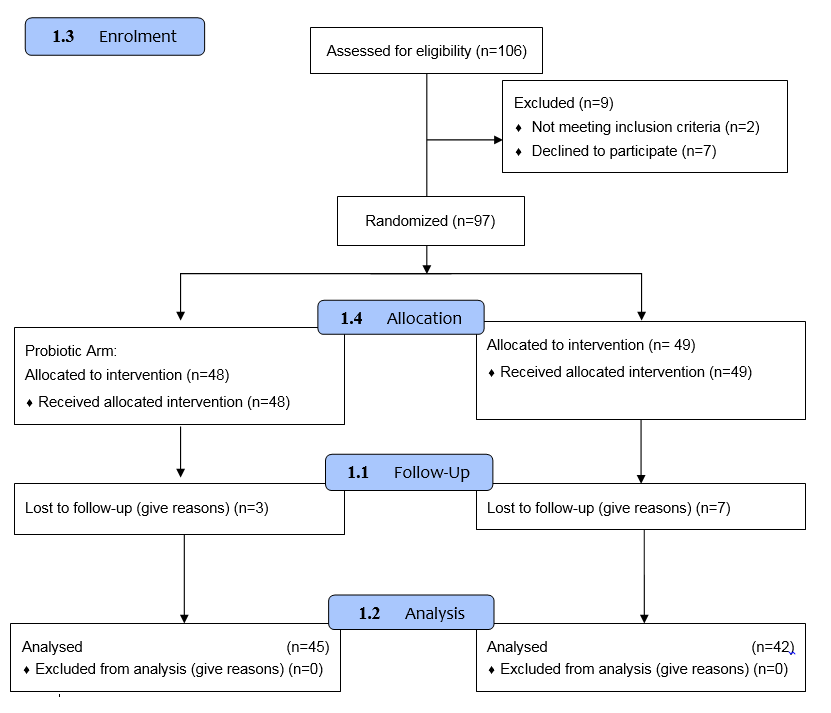
A total of 106 patients were initially enrolled in the study. Of these, 2 patients (1.88%) were excluded due to a lack of response to the medication, and 7 patients (6.60%) voluntarily withdrew from participation. Subsequently, 97 patients were randomized into two groups: the experimental group (n = 48) and the control group (n = 49). The analysis focused on evaluating itch severity score, hives severity score, and urticaria control test outcomes. During the follow-up period, 10 patients (10.39%) were lost to follow-up, including 3 from the experimental group and 7 from the control group. Consequently, the final analysis was conducted on 87 patients, comprising 45 patients in the experimental group and 42 in the control group ([Figure 1]).
|
Itch Severity Score |
Itch severity assessment |
Hives severity score |
Number of hives at each assessment |
|
0 |
None |
0 |
None |
|
1 |
Mild, minimal awareness, easily tolerated |
1 |
0-20 |
|
2 |
Moderate, definite awareness, bothersome but tolerable |
2 |
21-50 |
|
3 |
Severe, difficult to tolerate |
3 |
>50 |
|
S.No. |
Question |
Options |
||||
|
1 |
How much have you suffered from the physical symptoms of the urticaria (itch, hives, welts and/or swelling) in last four weeks |
Very much |
Much |
Somewhat |
A little |
Not at all |
|
2 |
How much was your quality of life affected by the urticaria in the last 4 weeks |
Very much |
Much |
Somewhat |
A little |
Not at all |
|
3 |
How often was the treatment for your urticaria in the last 4 weeks not enough to control your urticaria symptoms |
Very often |
Often |
Sometimes |
Seldom |
Not at all |
|
4 |
Overall, how well have you had your urticaria under control in the last 4 weeks |
Not at all |
A little |
Somewhat |
Well |
Very well |
|
Visits |
Control group meant rank |
Experimental group mean rank |
|
Visit-1 |
294.50 |
307.67 |
|
Visit-2 |
202.8 |
196.93 |
|
Visit-3 |
134.54 |
110.17 |
|
Visit-4 |
73.79 |
76.10 |
|
Visits |
Control group mean rank |
Experimental group mean rank |
|
Visit-1 |
281.18 |
270.50 |
|
Visit-2 |
173.56 |
268.39 |
|
Visit-3 |
84.60 |
150.37 |
|
Visit-4 |
73.50 |
88.23 |
|
Visits |
Control group meant rank |
Experimental group mean rank |
|
Visit-1 |
59.45 |
52.98 |
|
Visit-2 |
134.75 |
154.71 |
|
Visit-3 |
233.75 |
236.79 |
|
Visit-4 |
261.50 |
261.50 |
Analysis of itch severity score (ISS)
A Kruskal-Wallis test revealed significant differences in itch severity between control and experimental groups across four visits (H = 279.649, p < 0.0001). The experimental group exhibited the highest mean rank at Visit 1 (307.67), while the control group showed the lowest at Visit 4 (73.79). Post-hoc Mann-Whitney tests confirmed significant differences in ISS between control and experimental groups at Visit 1 (p = 0.01) but no significant differences at Visits 2, 3, and 4. Within-group analyses using Friedman and Wilcoxon tests demonstrated significant reductions in ISS across visits for both control (Chi² = 114.308, p < 0.0001) and experimental groups (Chi² = 123.424, p < 0.0001) ([Table 3]). Thus, the experimental group showed better control of itch severity during Visits 2 and 3, followed by a marked decrease at Visit 4, while the control group exhibited a steady decline from Visit 1 to Visit 4.
Analysis of hives severity score (HSS)
A Kruskal-Wallis test identified significant differences in HSS across visits for both groups (H = 265.712, p < 0.0001). At baseline (Visit 1), the control group had higher HSS, but the experimental group showed increased severity at later visits (2, 3, and 4). Friedman and Wilcoxon tests indicated significant reductions in HSS across visits within both groups. Notably, Visit 4 vs. Visit 3 comparisons showed borderline significance in the control group (p = 0.046) and non-significance in the experimental group (p = 0.157) ([Table 4]).
Analysis of urticaria control test (UCT)
The Kruskal-Wallis test revealed significant differences in UCT scores across visits for both groups (p < 0.0001). However, Mann-Whitney post-hoc tests showed no significant differences between the control and experimental groups at any visit (p > 0.05). Friedman and Wilcoxon tests indicated significant improvements in UCT scores across visits within each group ([Table 5]).
Thus, both the control and experimental groups exhibited significant within-group improvements in ISS, HSS, and UCT scores over time. The experimental group had transiently better itch control in intermediate visits, but the control group demonstrated more consistent reductions in hives severity. No significant differences in UCT scores were observed between the groups, suggesting comparable outcomes for overall urticaria control.
Discussion
Chronic spontaneous urticaria (CSU) is an immune-mediated dermatological condition characterized by recurrent wheals and/or angioedema in the absence of an identifiable trigger. The pathogenesis of CSU is associated with immune dysregulation involving mast cell activation, histamine release, and autoimmune mechanisms. Probiotics have been identified as potential modulators of the immune system in CSU through several mechanisms. First, they influence the gut-immune axis via the gut-associated lymphoid tissue, addressing dysbiosis linked to immune dysregulation and reducing systemic inflammation. Second, probiotics regulate immune cells, including T-cells, B-cells, and regulatory T-cells (Tregs), promoting immune tolerance and decreasing autoantibody production (e.g., IgE, IgG) involved in CSU. Third, they stabilize mast cells, mitigating excessive histamine release and alleviating clinical symptoms. Fourth, probiotics modulate cytokine profiles by enhancing anti-inflammatory mediators (e.g., IL-10) and suppressing pro-inflammatory cytokines (e.g., IL-4, IL-5, TNF-α), reducing chronic inflammation. Additionally, probiotics improve skin barrier function, limiting permeability and subsequent inflammatory responses, while also attenuating autoimmune activity by restoring gut homeostasis and regulating immune tolerance. These effects, however, are strain-specific and subject to individual variability, necessitating further research to optimize probiotic interventions in CSU management.[10] This study utilized multi-strain probiotics alongside conventional therapies to evaluate their efficacy in mitigating disease severity. The significant improvement in itch severity observed in the experimental group may be attributed to immunological modulation, including stabilization of immune dysregulation, inhibition of mast cell activation, reduction in histamine release, and regulation of autoimmune mechanisms.
Bi XD et al. conducted a randomized, placebo-controlled trial to evaluate the efficacy of probiotics as an adjunct therapy in children with chronic spontaneous urticaria (CSU). This study evaluated a six-strain probiotic (five Lactobacillus and one Bifidobacterium) administered orally twice daily for four weeks in children with chronic urticaria. The probiotics likely improved outcomes by modulating Treg cell function, enhancing intestinal microbiota composition, reducing pH, strengthening epithelial barrier integrity, regulating mucus secretion, and altering tight junction protein function. Combined with antihistamines, the probiotics significantly reduced symptom scores, wheal size, and attack frequency, supporting their use as adjunct therapy in school-age children with chronic urticaria. [11], [12], [13], [14], [15], [16], [17] We conducted a randomized, double-blind, placebo-controlled trial over 12 weeks in adults with chronic spontaneous urticaria (CSU) to evaluate the efficacy of probiotics as an adjunct to conventional therapy. Our study also demonstrated significant improvements in symptom scores, wheal size, and attack frequency, supporting the use of probiotics alongside conventional treatment to reduce disease severity.
Conclusion
Alterations in the gut microbiome and dysregulated Th1/Th2/Th17 cytokine responses are hypothesized to contribute to the pathogenesis of chronic spontaneous urticaria (CSU), with evidence from metabolomics revealing enteric dysbacteriosis in CSU patients. Gut microbiota may play a protective role and serve as probiotics for CSU management.[12] Our study demonstrated that probiotics, through immunological modulation, provide an additive effect in reducing disease severity in CSU patients. The immunomodulatory properties of probiotics provide a promising avenue for future therapeutic strategies in CSU management. Further large-scale, multi-center studies are warranted to validate these findings and explore underlying mechanisms.
Clinical Trial Registry Number
CTRI/2023/06/054428.
Sources of Funding
Velbiom Probiotics Bengaluru, Karnataka (DYPU/CIDR/Dermat/2023/01).
Conflict of Interest
None.
Acknowledgement
Velbiom Probiotics, Bengaluru Karnataka for providing Lactogut, multi-strain probiotics for the study.
References
- L Fu, BJ Cherayil, H Shi, Y Wang, Y Zhu. ood Allergy and the Microbiota: Implications for Probiotic Use in Regulating Allergic Responses. Food Allergy 2019. [Google Scholar] [Crossref]
- A Rezazadeh, S Shahabi, M Bagheri, E Nabizadeh, NH Jazani. The protective effect of Lactobacillus and Bifidobacterium as the gut microbiota members against chronic urticaria. Int Immunopharmacol 2018. [Google Scholar] [Crossref]
- E Nabizadeh, NH Jazani, M Bagheri, S Shahabi. Association of altered gut microbiota composition with chronic urticaria. Ann Allergy Asthma Immunol 2017. [Google Scholar]
- T Lu, Y Chen, Y Guo, J Sun, W Shen, M Yuan. Altered Gut Microbiota Diversity and Composition in Chronic Urticaria. Dis Markers 2019. [Google Scholar] [Crossref]
- E Nettis, Di Leo, E Pastore, A Distaso, M Zaza, I Vacca. Probiotics and refractory chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol 2016. [Google Scholar]
- L Paparo. Targeting Food Allergy with Probiotics. Probiotics and Child Gastrointestinal Health. Advances in Experimental Medicine and Biology 2019. [Google Scholar]
- K Hollis, C Proctor, D Mcbride, MM Balp, L McLeod, S Hunter. Comparison of Urticaria Activity Score Over 7 Days (UAS7) Values Obtained from Once-Daily and Twice-Daily Versions: Results from the ASSURE-CSU Study. Am J Clin Dermatol 2018. [Google Scholar]
- I Baiardini, M Pasquali, F Braido, F Fumagalli, L Guerra, E Compalati. A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-QoL). Allergy 2005. [Google Scholar]
- A Młynek, M Magerl, M Hanna, S Lhachimi, I Baiardini, GW Canonica. The German version of the Chronic Urticaria Quality-of-Life Questionnaire: factor analysis, validation, and initial clinical findings. Allergy 2009. [Google Scholar]
- M Krišto, L Lugović-Mihić, M Muñoz, M Rupnik, A Mahnic, P Ozretić. Gut Microbiome Composition in Patients with Chronic Urticaria: A Review of Current Evidence and Data. Life (Basel) 2023. [Google Scholar] [Crossref]
- XD Bi, BZ Lu, XX Pan, S Liu, JY Wang. Adjunct therapy with probiotics for chronic urticaria in children: randomised placebo-controlled trial. Allergy Asthma Clin Immunol 2021. [Google Scholar] [Crossref]
- R Cai, C Zhou, R Tang, Y Meng, J Zeng, Y Li. Current insights on gut microbiome and chronic urticaria: progress in the pathogenesis and opportunities for novel therapeutic approaches. Gut Microbes 2024. [Google Scholar] [Crossref]
- HY Fu, HD Yu, YP Bai, LF Yue, HM Wang, LL Li. Effect and safety of probiotics for treatingurticaria: A systematic review and meta-analysis. J Cosmet Dermatol 2023. [Google Scholar]
- N Atefi, M Fallahpour, S Sharifi, M Ghassemi, M Roohaninasab, A Goodarzi. Probiotic as an adjuvant therapy in chronic urticaria: a blinded randomized controlled clinical trial. Eur Ann Allergy ClinImmunol 2022. [Google Scholar]
- E Nettis, ED Leo, A Pastore, M Distaso, I Zaza, M Vacca. Probiotics and refractory chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol 2016. [Google Scholar]
- K Kukkonen, E Savilahti, T Haahtela, K Juntunen-Backman, R Korpela, T Poussa. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: randomized, double-blind, placebo-controlled trial. Pediatrics 2008. [Google Scholar]
- TR Abrahamsson, T Jakobsson, MF Bottcher, M Fredrikson, MC Jenmalm, B Björkstén. Probioticsin prevention of IgE- associated eczema: a double- blind, ran-domized, placebo- controlled trial. J Allergy Clin Immunol 2007. [Google Scholar]
