- Visibility 64 Views
- Downloads 3 Downloads
- DOI 10.18231/2581-4729.2019.0011
-
CrossMark
- Citation
A study on serum ig E and AEC in chronic urticaria and correlation between serum ig E and disease activity in chronic urticaria
- Author Details:
-
Puneetha B
-
Naveen N *
Abstract
Introduction: Urticaria is one of the most common allergic skin disorders having significant psychological impact. It occurs either acutely or evolves in chronic course, this study estimates significance of serum IgE levels, AEC in chronic urticaria and also the association between activity of chronic urticaria and serum Ig E levels.
Objective: To measure the serum Ig E levels and Absolute eosinophil count (AEC) in cases and control group and to correlate Serum Ig E with disease activity in chronic urticaria
Materials and Methods: It is a prospective case control study conducted in 103 chronic urticaria cases, Serum Ig E levels and AEC were measured in cases and controls, serum Ig E level was correlated with UAS7 in cases.
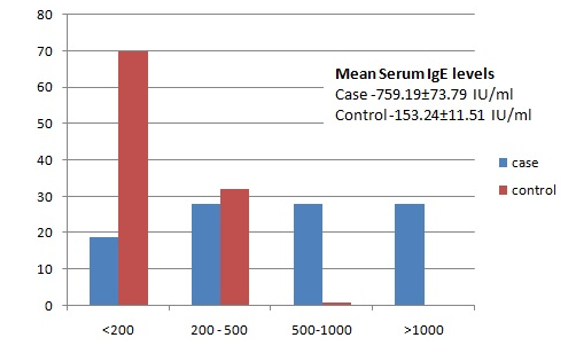
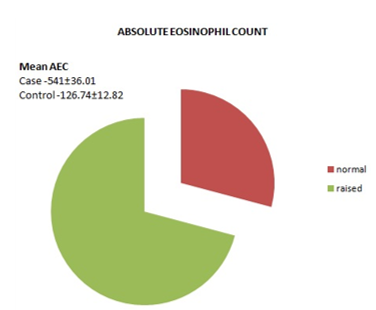
Results: Out of 103 age and sex matched cases and controls the mean age was 29.23±1.27 years in patient group, mean AEC in patients was 541±36.01, significantly higher compared to the controls 126.74±12.82, and mean Ig E levels in patients was 759.19±73.79 IU/ml, significantly higher compared to the controls 153.24±11.51IU/ml.
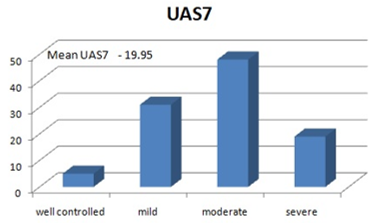
Mean UAS7 score was 19.95±1.02 in cases, five had well controlled urticaria (4.85%), 31 mild (30.09%), 48 moderate (46.60%) and 19 had severe urticaria (18.44%). We observed a statistically significant correlation between serum Ig E and UAS7.
Conclusion: The present study suggests that serum Ig E levels needs to be considered as a valuable serological marker of disease severity in cases of chronic urticaria or management. Further similar studies are also required to assess based on the regional and ecological changes as the immunology differs from region to region and lifestyle changes.
Keywords: Chronic urticaria, Serum Ig E levels, AEC, UAS7.
Introduction
Urticaria is a common allergic skin disease affecting 15% to 25% of the population atleast once in lifetime, which occurs either acutely or evolve in chronic course. Acute urticaria can present as acute onset of transient wheals with or without associated angioedema for less than 6 weeks, whereas chronic urticaria presents with wheals occurring continuously or intermittently for at least 6 or more weeks.[1],[2] Urticaria has a major psychological impact on the quality of life of patients. The present study has been undertaken to estimate the significance of association between activity of chronic urticaria, serum IgE levels and absolute eosinophil count.
On review of the literature, studies show variable association between IgE levels and AEC in cases and controls which is contradictory; in the present prospective study we demonstrate that chronic urticaria patients have significant association with Serum IgE and Urticaria disease severity. These results indicate that IgE levels are a sensitive marker of urticaria severity.
Objective
- To measure the serum IgE levels and Absolute eosinophil count (AEC) in cases and control group.
- To correlate Serum IgE with disease activity in chronic urticaria.
Materials and Methods
A prospective case control study was conducted in a tertiary care hospital in southern India, dermatology department involving 103 patients diagnosed with chronic urticaria. 103 age and sex matched controls were included and individuals with personal history of atopy were excluded in controls. Prior written consent was taken before enrolment from both cases and controls.
All participants were subjected to the following:
- Information regarding the duration of disease, pruritus, number and size of wheals and the number of days with urticaria in a week were noted to grade the urticaria based on urticaria activity score. For uniformity all cases were graded based on UAS 7 as given in table 1.
- Laboratory investigations
- Venous blood samples were collected in k2 EDTA tube and Complete blood count with absolute eosinophil count were obtained by sysmex 5 part hematology analyzer, a range 30-350 was considered as normal AEC.
- Serum Ig E levels were estimated through immunoassay using Immunocap 250 system in all cases. Normal range of Ig E given in table 2.
Statistical analysis
Comparative analysis of patient and control groups was performed using the SPSS software, version 16. Association between patient and control serum Ig E and AEC was done by paired t test and for association within the patient group independent t test and spearman’s rho test was used as needed. Two tailed p values <0>
Results
A total of 103 cases of chronic urticaria of which 61 males and 42 females were involved, similar age and sex matched controls were involved in the study. The mean age of cases was 29.23±1.27 years (3 months to 62 years). Amongst which 23 patients (22.33%) were of paediatric age group (<18>
Association of Ig E with UAS7: Out of the 67 cases of moderate to severe urticaria 100% had raised Ig E levels. There was also a specific significant association between Serum Ig E levels and UAS7 scoring (p value <0>
Table 1: Urticaria activity score 7.
|
UAS7 Score |
Health state |
|
0 |
Urticaria free |
|
1-6 |
Well controlled urticaria |
|
7-15 |
Mild urticaria |
|
16-27 |
Moderate urticaria |
|
28-42 |
Severe urticaria |
Table 2: Normal Serum Ig E levels.
|
Normal Ig E level |
IU/ml |
|
Neonates |
Upto 1.5 |
|
<1> |
15 |
|
1-5 |
60 |
|
6-9 |
90 |
|
10-15 |
200 |
|
>15 |
100 |
Table 3: Age of cases.
|
Age |
Frequency |
Percent(%) |
|
<1y> |
1 |
0.97 |
|
1-5y |
3 |
2.91 |
|
6-10y |
4 |
3.88 |
|
11-18y |
13 |
12.62 |
|
19-25y |
20 |
19.41 |
|
26-35y |
29 |
28.15 |
|
36-65y |
33 |
32.03 |
|
Total |
103 |
100.0 |
Table 4: UAS7 scoring in cases.
|
UAS7 |
Scoring |
Number of cases |
Percent (%) |
|
Well controlled |
1-6 |
5 |
4.85 |
|
Mild |
7-15 |
31 |
30.09 |
|
Moderate |
16-27 |
48 |
46.60 |
|
Severe |
28-42 |
19 |
18.44 |
|
|
Total |
103 |
100 |
Table 5: Correlation of serum Ig E and UAS7.
|
Correlations |
|||||
|
|
IGE |
UAS |
AEC |
||
|
Spearman's rho |
IGE |
Correlation Coefficient |
1.000 |
.996** |
.999** |
|
Sig. (2-tailed) |
. |
.000 |
.000 |
||
|
N |
103 |
103 |
103 |
||
|
UAS |
Correlation Coefficient |
.996** |
1.000 |
.995** |
|
|
Sig. (2-tailed) |
.000 |
. |
.000 |
||
|
N |
103 |
103 |
103 |
||
|
AEC |
Correlation Coefficient |
.999** |
.995** |
1.000 |
|
|
Sig. (2-tailed) |
.000 |
.000 |
. |
||
|
N |
103 |
103 |
103 |
||
|
**. Correlation is significant at the 0.01 level (2-tailed). |
|||||
Table 6: Comparison of mean Serum Ig E with other studies.
|
Mean Serum Ig E in cases |
Present study |
Kessel A et al[3] |
Zaky A et al[4] |
Tang et al[5] |
Yilmaz EA et al[6] |
|
IU/ml |
759.19±73.79 |
182±16.12 |
494.27 U |
159.58 ±4.43 |
63.5 |
Table 7: Comparison of UAS7 with other studies.
|
|
Present study |
Yilmaz EA et al[7] |
Tang Ni Et al[8] |
Emma H et al[9] |
|
|
Mean UAS7 Score |
19.95 |
28 |
21 |
31.1 |
36.3 ± 5.4 |
 |
Click here to view |
Fig. 1: Serum IgE levels of case and controls.
 |
Click here to view |
Fig. 2: Absolute eosinophil count in cases and controls.
 |
Click here to view |
Fig. 3: Urticaria Activity Score 7.
Discussion
Urticaria is a common allergic skin disease affecting 15% to 25% of the population atleast once in lifetime, which occurs either acutely or evolve in chronic course.[1]Acute urticaria can present as acute onset of transient wheals with or without associated angioedema for less than 6 weeks, whereas chronic urticaria presents with wheals occurring continuously or intermittently for atleast 6 or more weeks.[2] Urticaria has a major psychological impact on the quality of life of patients. Chronic urticaria (CU) affects 0.5–1% of the general population worldwide with CSU accounting for more than two-thirds of CU cases.[11] Urticaria is a mast cell-driven disease. Histamine and other mediators, such as platelet-activating factor (PAF) and cytokines released from activated skin mast cells, result in sensory nerve activation, vasodilatation and plasma extravasation as well as cell recruitment to urticarial lesions. The mast cell-activating signals in urticaria are ill-defined and likely to be heterogeneous and diverse. Highly selective ligand interactions ELAM 1 and ICAM 1 provide a powerful mechanism to control the sequential evolution of inflammatory cell infiltration in urticarial wheals during their development in time.[12]Mast cells (MC) are the primary effector cells in urticaria and in many cases of angioedema. These cells are widely distributed in the skin, mucosa, and other areas of the body, and have high-affinity immunoglobulin E (IgE) receptors.[1] Immunoglobulin E is believed to be one of the major mediators of immediate hypersensitivity reactions that underlie atopic conditions such as urticaria[13] IgE-mediated allergic mechanisms are incriminated in certain cases of chronic urticaria, because elevated serum IgE levels are found in these patients.[14] Previous studies have shown serum IgE levels and AEC to be significantly elevated in the urticaria patients as compared with healthy volunteers. The mast cells release eosinophil chemotactic factors which attract eosinophils to the sites of anaphylaxis, urticaria, angioedema, and atopy.[15] The increased expression and density of Fc?RI on mast cells from patients with CU following the elevation of IgE renders these cells more sensitive to the binding of autoantibodies against Fc?RI.[3] The anti inflammatory effect of omalizumab and its possible clinical benefit for CU patients emphasizes the fundamental importance of serum IgE and its association with urticaria severity. The role of tissue eosinophilia is unclear, although it is possible that release of toxic major basic protein and eosinophil cationic protein further augments histamine release from mast cells in the late phase of the urticarial wheal.[16]Eosinophils are recruited following the release of cytokines and chemotactic factors and activation and recruitment of adhesion molecules on migrating eosinophils and on endothelial cells.[17] Eosinophil-mediated activation of the coagulation system may be another mechanism of MC degranulation in patients, who have both CU and Parasitic infestations.(PI).[18] The prevalence of PI in CSU ranges from 0% to 38.8% (Turkey), 3.3% to 37.8% (India), and 0.7% to 10.5% (Thailand). Recent studies conducted in India have reported the prevalence of intestinal PI ranging from 60% to 91%.[19]The most common parasites were Giardia intestinalis (62%), Enterobius vermicularis (16%), Ascaris lumbricoides (7%), and Blastocystis hominis (6%).[20]Currently, the laboratory gold standard test for CU is basophil degranulation stimulation test, a difficult to perform and costly in vitro test, used only in major research center.[21] ,[16]Other investigations done are RAST (Radioallergosorbent test), RA factor, ANA, Autologous serum skin test (ASST), Anti TPO antibodies, Complement analysis, Cryoglobulin levels, Skin biopsy, G6PD enzyme deficiency.[8]In developing countries with limited resources common investigations done in urticaria patients are Complete blood count with differential count, Absolute Eosinophil Count, Serum Ig E levels considering the cost efficacy, as compared with other investigations.
Urticaria Activity Score (UAS) assesses daily pruritus and number of hives, which summed over a week, gives UAS7 score (range: 0–42) with a higher score corresponds to higher disease severity. The UAS7 score is commonly used to assess the CU disease activity[22]and has been recommended by guidelines for routine clinical practice to determine disease activity and response to treatment.[23] UAS7 was considered as it was directly correlating with Dermatology quality of life index (DLQI) [23] and a simple and easy protocol to grade patients for treatment as well as research purposes. In present study 66% of CU cases had moderate to severe Urticaria as compared to 77% in Kessel A et al.8 and 64.29% in Zaky et al study.[4]
The mean serum IgE levels were 759.19±73.79 IU/ml in the present study. Other similar studies by Zaky A et al.[4] Tang et al.[5]and Yilmaz EA et al.[7] showed variable differences in serum IgE levels (Table 6). The mean UAS7 in our study was 19.95, other studies by Yilmaz EA et al.[7]Tang Ni Et al.[5] Emma H et al.[6]and Pinto G M et al.[9]showed variable values as given in table 7
The significant difference may be attributed to the regional factors contributing to the immunity of the patient, genetic makeup and age of the patient. Environmental factors which contribute range from abiotic chemicals exposures and nutritional status to biotic insult from infectious diseases and with microbial or parasitic colonization. Early life represents vulnerability and opportunity which impact the developing immune system. These include potential inherited programming (genetic and epigenetic), derailed development due to altered metabolism (nutrition and toxicology), and inappropriate immune system decision-making (for both adaptive and innate arms).[10]
Conclusion
There are several studies related to Ig E levels and urticaria activity, further the present study implicates that Ig E levels should be considered as a marker in evaluating CU patients to assess disease severity, perhaps affecting patient management. Further similar studies are also required to assess based on the regional and ecological changes as the immunology differs from region to region and lifestyle changes.
Conflicts of Interest: None.
References
- a, b, c Amar SM, Dreskin SC. Urticaria. Prim Care. 2008;35(1):141–157.
- a, b Poonawalla T, Kelly B. Urticaria: a review. Am J Clin Dermatol 2009;10(1):9–21.
- a, b Kessel A et al. Elevated Serum Total IgE – A Potential Marker for Severe Chronic Urticaria. Int Arch Allergy Immunol 2010;153:288-293.
- a, b, c Zaky A, Khalifa S, Mohsen AR. Chronic idiopathic urticaria and atopy, is there any relation?. Gulf J dermatol venerol 2010,7:32-35.
- a, b, c Tang Ni, Man-Yun M, Rui Z, Xiang C, Jiang-Lin Z, Wu Z, Jie L. Clinical characteristics of urticaria in children versus adults. CJCP.2017;19(7):790-795.
- a, b Emma H et al. EQ-5D Utilities in Chronic Spontaneous/Idiopathic Urticaria. PharmacoEconomics 2016;34:521–527.
- a, b, c Yilmaz EA.The persistence of chronic spontaneous urticaria in childhood is associated with the urticaria activity score. Allergy Asthma Proc 2017;38:136-142.
- a, b Randy D, Danielsen, Emeritus, Gabriel Ortiz, Susan Symington, Chronic Urticaria It’s More Than Just Antihistamines! Clin rev 2018:36-42.
- a, b Pinto G M, Gameiro A, Pinho A, Gonçalo M. Long-term management of chronic spontaneous urticaria with omalizumab. Clin Exp Dermatol 2017Oct;42(7):735-742.
- a, b Duncun M, Gillivrey M, Kollman TR. The Role of Environmental Factors in Modulating Immune Responses in Early Life. Front Immunol 2014;5:434.
- ^ Maurer M, Weller K, Bindslev-Jensen C, Gimenez-Arnau A, Bousquet PJ, Bousquet J et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy 2011;66(3):317–330.
- ^ Haas, N, Schadendorf, D, & Henz B. M. Differential Endothelial Adhesion Molecule Expression in Early and Late Whealing Reactions. Int Arch Allergy Immunol 1998;115(3):210–214.
- ^ Corry DB, Kheradmund F, Induction and regulation of the IgE response Nature.1999;402:18- 23.
- ^ Schocket AL. Chronic urticaria: pathophysiology and etiology, or the and why. Allergy Asthma Proc 2006;27:90-95.
- ^ Jenerowicz D, Czarnecka, Operacz M, Silny W. Peripheral blood eosinophilia in atopic dermatitis. Acta Dermatovenerol Alp Pannonica Adriat 2007;16:47-52.
- a, b Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza BA, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol 1999;140:446-452.
- ^ Lee KH, Kim JY, Kang DS, Choi YJ, Lee WJ, Ro JY. Increased expression of endothelial cell adhesion molecules due to mediator release from human fore skin mast cells stimulated by autoantibodies in chronic urticaria sera. J Invest Dermatol 2002;118:658-663.
- ^ Tedeschi A, Kolkhir P, Asero R, Pogorelov D, Olisova O, Kochergin N et al. Chronic urticaria and coagulation: pathophysiological and clinical aspects. Allergy 2014;69:683–691.
- ^ Fernandez MC, Verghese S, Bhuvaneswari R, Elizabeth SJ, Mathew T, Anitha A et al. A comparative study of the intestinal parasites prevalent among children living in rural and urban settings in and around Chennai. J Commun Dis 2002;34:35–39.
- ^ Kirkoyun UH, Akgul O, Purisa S, Oner YA. Twenty-five years of intestinal parasite prevalence in Istanbul University, Istanbul Faculty of Medicine: a retrospective study. Turkiye Parazitol Derg 2014;38:97–101.
- ^ Platzer MH, Grattan CEH, Poulsen LK, Skov OS. Validation of basophil histamine release against the autologous serum skin test and outcome of serum-induced basophil histamine release studies in a large population of chronic urticarial patients. Allergy 2005; 60(9): 1152-1156.
- ^ Mlynek A et al. How to assess disease activity in patients with chronic urticaria? Allergy 2008;63(6):777–780.
- a, b Zuberbier T et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy 2009;64(10):1417–1426.
