- Visibility 231 Views
- Downloads 27 Downloads
- DOI 10.18231/j.ijced.2021.023
-
CrossMark
- Citation
A real world retrospective analysis of comparison of effectiveness and safety of mometasone furoate and fluticasone propionate in the management of eczema and dermatitis
- Author Details:
-
R D Kharkar
-
Dhiraj Dhoot *
-
Harshal Mahajan
-
Hanmant Barkate
Introduction
Dermatitis is a general term for conditions that causes inflammation of the skin. There are many different types dermatitis like contact dermatitis, atopic dermatitis etc. Contact dermatitis is one of the commonest type of dermatitis and accounts for 70-90% of all occupational skin diseases.[1], [2] It is an inflammatory skin condition induced by exposure to an external irritant or allergen. A prevalence of 8.2% was seen in a recent cross sectional study of 12377 subjects across five European countries, in which a randomly selected group of 3119 patients were patch tested. [3] The condition can have a detrimental impact on personal and social relationships, quality of life, and even threaten employment. [4], [5], [6]
Along with emollients the local treatment of choice is a topical corticosteroid. Topical corticosteroids are a family of hydrocortisone derivative compounds with variable anti-inflammatory potency and side-effects profile. Their relative potency should be carefully considered when choosing them for treating individual patients. While the least potent corticosteroids may be sufficient in certain conditions, clinical settings, and for long-term maintenance therapy, the same medication may be ineffective in some other conditions. [7] In such conditions, mid to high potent corticosteroids are preferred. These agents are very effective in the short term in contact dermatitis. [8] Apart from this, once daily treatment is sufficient and may even be superior to twice daily application. [9]
Currently, Mometasone furoate (0.1%) and fluticasone propionate (0.005%) are commonly prescribed in contact dermatitis as either once a day or twice a day application respectively. But there is no comparative data between these two drugs. Hence we conducted this retrospective data analysis to compare clinical assessment between these two drugs in contact dermatitis.
After obtaining ethics committee approval, a retrospective data analysis of medical records was carried out at 236 centres across India, to compare the effectiveness and tolerability of mometasone 0.1% cream and fluticasone 0.005% cream in the management of contact dermatitis during September 2020 to January 2021. According to patients’ clinical records, assessments were done at baseline and 2 weeks, using reduction in total symptom score and physician and patient assessment.
We considered oedema, pruritus, erythema and lichenification for calculating total symptom score (TSS). Each symptom was graded from 0-3 as none to severe category. Based on TSS after 2 weeks of treatment, patients were categorized as remarkably improved (TSS=0), improved (reduction in TSS >50% of baseline value), or no/minimal improvement (no/ less than 50% reduction in TSS as compared to baseline value). Response was defined based on the follow-up assessment at 2 weeks as compared with the baseline evaluation. Tolerability was evaluated by assessing the incidence of treatment emergent adverse events (TEAEs), treatment related AEs and AEs/SAEs leading to study withdrawal in both the treatment groups.
Binomial variables were expressed as number & percentage and continuous variables as mean (S.D.). Paired t test was used for comparisons between baseline and follow-up measurements. Significant differences between responders and non-responders were defined as those at a level of p<0.05 by paired t test.
Results
Out of 1706 patients, out of which 1106 patients’ data fulfilled inclusion criteria and were considered for final analysis. Baseline demographics are depicted in [Table 1]. On further analysis, we found aggravating factors for dermatitis which are depicted in [Table 2].
|
|
Mometasone |
Fluticasone |
P value |
|
N |
598 |
508 |
|
|
Male (%) |
294 (49) |
248 (49) |
|
|
Female (%) |
304 (51) |
260 (51) |
|
|
Age years (SD) |
33.66±11.08 |
32.94±13.06 |
0.32 |
|
Predisposing factors; N (%) |
|||
|
Artificial Jewellery |
119 (20) |
130 (26) |
|
|
Detergent |
186 (31) |
136 (27) |
|
|
Use of Sanitizers |
153 (25) |
113 (22) |
|
|
Occlusive foot ware |
101 (17) |
86 (17) |
|
|
No history |
39 (7) |
43 (8) |
|
|
Severity of Dermatitis; N (%) |
|||
|
Mild |
37 (6) |
47 (9) |
|
|
Moderate |
369 (62) |
331 (65) |
|
|
Severe |
192 (32) |
130 (26) |
|
|
Mean total symptom score |
7.43±2.08 |
7.29±2.13 |
0.27 |
|
Physician global assessment |
2.89±0.95 |
2.87±0.83 |
0.71 |
|
Patient assessment |
3.05±0.99 |
2.97±0.86 |
0.15 |
|
|
Baseline TSS |
||
|
Mometasone |
Fluticasone |
p value |
|
|
Artificial jewellery |
7.50±2.23 |
7.04±2.06 |
0.09 |
|
Detergent |
7.40±1.93 |
7.61±1.95 |
0.33 |
|
Sanitizer |
7.70±2.11 |
7.29±2.06 |
0.11 |
|
Occlusive foot ware |
8.30±2.06 |
8.43±2.07 |
0.66 |
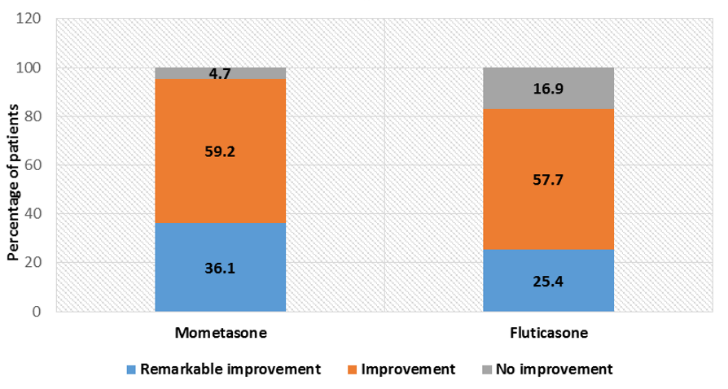
In mometasone group, 216 patients (36.1%) had TSS of zero whereas 129 patients (25.4%) in fluticasone group achieved the same. Mometasone group was found to be statistically significant than fluticasone group in complete clearance of symptoms (p<0.05) as shown in [Figure 1]. In mometasone group, 354 patients (59.2%) were found to achieve improvement whereas 293 patients (57.7%) achieved the same in fluticasone group. No or minimal improvement was found in 28(4.7%) and 86(16.9%) patients in mometasone group and fluticasone group, respectively. There was statistical difference between TSS at baseline and end of therapy in both the groups (p value <0.05). However on intergroup comparison, improvement in mometasone group was statistically significant than fluticasone group as shown in [Table 3].
|
Effectiveness parameters |
Mometasone |
Fluticasone |
p value |
|
Total Symptom Score |
1.34±1.43 |
2.13±1.90 |
0.0001 |
|
Physician global assessment |
0.72±1.29 |
0.85±0.75 |
0.045 |
|
Patient assessment |
0.71±1.51 |
0.92±0.80 |
0.004 |
At baseline, there were 37 patients in mild category in mometasone group whereas 47 patients in fluticasone group. Out of these, 29 patients were symptom free at the end of therapy (p=0.15). But there was statistical difference between both the groups in moderate and severe category as shown in [Table 4].
|
Severity of dermatitis |
Mometasone |
Fluticasone |
p value |
|
Mild |
29 |
29 |
0.15 |
|
Moderate |
140 |
89 |
0.01 |
|
Severe |
47 |
11 |
0.002 |
On further sub group analysis on aggravating factors, it was found that, Mometasone was statistically significant in relieving symptoms of dermatitis due to artificial jewellery, detergent and occlusive foot ware as shown in [Table 5].
|
|
End of therapy TSS |
||
|
Mometasone |
Fluticasone |
p value |
|
|
Artificial jewellery |
1.15±1.52 |
2.05±1.99 |
0.0001 |
|
Detergent |
1.29±1.21 |
2.41±1.91 |
0.0001 |
|
Sanitizer |
1.52±1.54 |
1.71±1.70 |
0.34 |
|
Occlusive foot ware |
1.48±1.64 |
2.49±2.15 |
0.0004 |
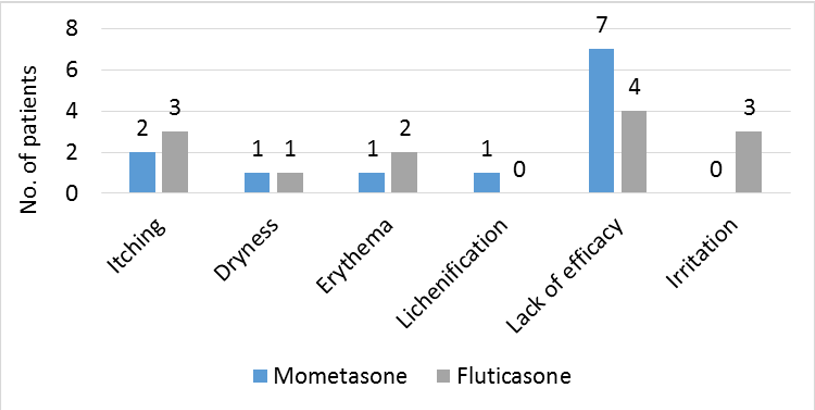
Both the treatments were safe and well tolerated by patients. A total of 12 patients reported AE in mometasone group and 13 in fluticasone group. There was no statistical difference in terms of safety and tolerability (p=0.2). Details of AEs are depicted in [Figure 2].
Discussion
Eczema/dermatitis is one of the commonest dermatological ailments.[10] Topical corticosteroids (TCS) are mainly used for management of these conditions. But there is increased propensity of adverse events (AEs) with the use of high potency TCS. Mometasone furoate cream is a medium potency TCS which will have comparatively less propensity of AEs and have reasonable efficacy.[11] The present survey will be first of its kind in India to provide evidence of real world effectiveness and safety of mometasone furoate and fluticasone in treatment of eczema/dermatitis.
In the present survey, it was found that the effect of mometasone furoate was significantly more as compared to fluticasone propionate, as indicated by total symptom score, physician’ global assessment and patient assessment scores. In a randomized clinical trial by Pei et al., the effectiveness of mometasone furoate 0.1% was compared with fluticasone propionate 0.05%. On comparison of extent of disease score at the end of therapy, there was reduction by 48 points in mometasone furoate group as compared to 30 points reduction in fluticasone propionate group. Both the treatment groups showed significant improvement under wet wrap dressing with more improvement seen in mometasone furoate group.[12]
The efficacy and safety of mometasone furoate cream has been assessed on a large scale in various dermatitis and eczema through clinical trials. It has been found to be superior over hydrocortisone butyrate 0.05%, betamethasone valerate 0.1 %, hydrocortisone valerate 0.2 %, hydrocortisone 1.0 % in cases of atopic dermatitis. Thus, globally mometasone furoate has shown to be superior to placebo as well as active drugs in various settings. One of the established advantage of mometasone furoate over other topical corticosteroids is its once daily application to achieve the clinical effect.[11] This will help to increase the patient compliance, as well. [13]
Mometasone furoate possess anti-inflammatory and anti-pruritic actions. [14] The anti-inflammatory effect is mediated via binding of mometasone to its corticosteroid receptor and this complex causes suppression of genes responsible for inflammatory cytokine production. [15] The anti-inflammatory actions of mometasone furoate and fluticasone propionate were compared in patients with allergic rhinitis. In this study, it was found that both the treatment groups had significant improvement in total symptom score, but the improvement was more in mometasone group as compared to fluticasone group and the difference was highly statistically significant (p<0.001). It was inferred that mometasone furoate had better efficacy in relieving inflammatory symptoms of rhinitis as compared to fluticasone. [16] In the present study, more number of patients had shown remarkable improvement and greater reduction in severity of the disease. This can be attributed to better anti-inflammatory effect of mometasone furoate.
In the present study, it was found that use of occlusive footwear, artificial jewellery, sanitizer, detergents were commonest predisposing factors for dermatitis. Similar findings were reported in an observational Indian study by Thilak S et al. [17] Particularly, the indiscriminate use of alcohol based sanitizer in wake of COVID 19 pandemic has led to increased occurrence of contact dermatitis, wherein symptoms were resolved by use of topical corticosteroid, moisturizer and avoidance of sanitizer. [18], [19] Reduction in TSS in mometasone group was more as compared to fluticasone group in all these predisposing factors.
There was no significant difference in terms of safety profile in both the treatment groups. Once applied on the skin, ester hydrolysis biotransformation of mometasone reduces its binding affinity as it passes towards dermis. Thus, its increases the binding affinity to corticosteroid receptor in dermis is reduced as compared to epidermis and therefore the efficacy is maintained and at the same time the chances of undesirable effects are greatly reduced. [20]
Thus, mometasone furoate has shown good effectiveness and similar safety profile as compared to fluticasone in treatment of eczema/dermatitis. The present study is not without limitations. Due to its retroactive design the chances of bias cannot be ruled out. It is recommended that such studies should be carried out prospectively, as multiple centres so that results of present study can be compared, validated and generalised.
Conclusion
Both, mometasone furoate and fluticasone were effective and safe in treatment of eczema/dermatitis. But mometasone furoate had shown significantly better effectiveness as compared to fluticasone in all predisposing factors for the disease.
Acknowledgements
Authors would like to thank all the dermatologists who provided their data for this analysis.
Source of Funding
No financial support was received for the work within this manuscript.
Conflicts of Interest
There are no conflicts of interest.
References
- A Adisesh, E Robinson, P J Nicholson, D Sen, M Wilkinson. Standards of Care Working Group. U.K. standards of care for occupational contact dermatitis and occupational contact urticaria. Br J Dermatol 2013. [Google Scholar]
- RS Rashid, TN Shim. Contact dermatitis. BMJ 2016. [Google Scholar] [Crossref]
- T.L. Diepgen, R.F. Ofenloch, M. Bruze, P. Bertuccio, S. Cazzaniga, PJ Coenraads. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol 2016. [Google Scholar] [Crossref]
- DL Kadyk, K McCarter, F Achen, DV Belsito. Quality of life in patients with allergic contact dermatitis. J Am Acad Dermatol 2003. [Google Scholar] [Crossref]
- D Boehm, G Schmid-Ott, F Finkeldey, S Malte John, C Dwinger, T Werfel. Anxiety, depression and impaired health-related quality of life in patients with occupational hand eczema. Contact Dermat 2012. [Google Scholar] [Crossref]
- DL Holness, D Beaton, E Harniman, J DeKoven, S Skotnicki, R Nixon. Hand and Upper Extremity Function in Workers With Hand Dermatitis. Dermatitis 2013. [Google Scholar] [Crossref]
- S Molin, D Abeck, A Guilabert, M Bellosta. Mometasone Furoate: A Well-Established Topical Corticosteroid now with Improved Galenic Formulations. J Clin Exp Dermatol Res 2013. [Google Scholar]
- T L Diepgen, K E Andersen, O Chosidow, P J Coenraads, P Elsner, J English. Guidelines for diagnosis, prevention and treatment of hand eczema. J Dtsch Dermatol Ges 2015. [Google Scholar]
- M Loden, K Wiren, K T Smerud. The effect of a corticoste¬roid cream and a barrier-strengthening moisturizer in hand eczema. A double-blind, randomized, prospective, parallel group clinical trial. J Eur Acad Dermatol Venereol 2012. [Google Scholar]
- KY Sayaseng, P Vernon. Pathophysiology and Management of Mild to Moderate Pediatric Atopic Dermatitis. J Pediatr Health Care 2018. [Google Scholar]
- S Molin, D Abeck, A Guilabert. Mometasone Furoate: A Well-Established Topical Corticosteroid now with Improved Galenic Formulations. J Clin Exp Dermatol Res . [Google Scholar]
- AYS Pei, HHL Chan, KM Ho. The Effectiveness of Wet Wrap Dressings Using 0.1% Mometasone Furoate and 0.005% Fluticasone Proprionate Ointments in the Treatment of Moderate to Severe Atopic Dermatitis in Children. Pediatri Dermatol 2001. [Google Scholar] [Crossref]
- J Jin, G Sklar, V Min Sen Oh. Factors affecting therapeutic compliance: A review from the patient's perspective. Ther Clin Risk Manag 2008. [Google Scholar]
- S. Shaikh, M. S. Muneera, O. A. Thusleem, M. Tahir, A. V. Kondaguli. A Simple RP-HPLC Method for the Simultaneous Quantitation of Chlorocresol, Mometasone Furoate, and Fusidic Acid in Creams. J Chromatographic Sci 2009. [Google Scholar] [Crossref]
- EM King, JE Chivers, CF Rider, A Minnich, MA Giembycz, R Newton. Glucocorticoid Repression of Inflammatory Gene Expression Shows Differential Responsiveness by Transactivation- and Transrepression-Dependent Mechanisms. PLoS ONE 2013. [Google Scholar] [Crossref]
- W Ul Hamid, D Sumbria, I Ali, R Ahmad. A comparative study to assess the efficacy of fluticasone and mometasone in allergic rhinitis. Int J Otorhinolaryngol Head Neck Surg 2020. [Google Scholar] [Crossref]
- T Sundararaj, M Govindaraju, B Thangaraj. A study of 300 cases of allergic contact dermatitis. Int J Res Dermatol 2017. [Google Scholar] [Crossref]
- D Inder, P Kumar. Isopropyl alcohol (70%)- based hand sanitizer-induced contact dermatitis: A case report amid Covid-19. Indian J Case Reports 2020. [Google Scholar]
- V Pope, L Ousley. Irritant Contact Dermatitis Caused by Hand Sanitizer Use and Handwashing During the COVID-19 Pandemic. Consultant 2020. [Google Scholar] [Crossref]
- HC Korting, MJ Kerscher, M Schäfer-Korting. Topical glucocorticoids with improved benefit/risk ratio: Do they exist?. J Am Acad Dermatol 1992. [Google Scholar] [Crossref]
