- Visibility 1.4k Views
- Downloads 47 Downloads
- DOI 10.18231/j.ijced.2021.047
-
CrossMark
- Citation
A single arm, exploratory and pilot study to evaluate the efficacy of a multi-component water-based herbal supplement in hair growth, density, follicle strength and hair fall in healthy male and female subjects
- Author Details:
-
C.S Janaki *
-
Mukta Sachdev
-
Ritambhara
Introduction
Hair loss is a common problem worldwide, affecting around 50% of all males worldwide and a quarter of females. Hair loss may be interpreted as an abnormality and a failure to conform to societal standards of physical appearance, which has the potential to distinguish individuals in their own and others' estimations. Psychological and social stress on women is “immense” compared to men, which can contribute to hair loss among young women.
Various literatures have documented that hair loss or hair fall is one of the major concerns in men and women of all ages. This problem may start early and may increase significantly with age.[1], [2], [3], [4], [5]
Most available therapeutics to address alopecia are based on singular targets and mechanism of action based on the assumption of sub-classification to reflect morphological or etiological sub-classes such as hereditary vs acquired factors, anti-inflammatory vs. non-inflammatory factors or scarring vs. non-scarring factors. Accordingly, most therapeutic interventions are based on antiandrogen medications also known as androgen antagonists or testosterone blockers (Finasteride, Dutasteride) and vasodilator minoxidil stimulatory effect on hair growth hypothesized to be from the opening of potassium channels by minoxidil sulphate.[6]
Unfortunately, the hair follicle life cycle is a complex process involving four main phases of anagen (growth), catagen (regression), telogen (rest), and exogen (shedding). Each of these phases is impacted by anatomical location, nutritional and hormonal status, age, and environmental factors such as stress.[7] These processes therefore represent a complex, multi-factorial mechanisms where multiple perturbations impact the hair follicle life cycle.
In order to address the complexities associated with hair fall and hair thinning, a novel nutraceutical-based supplement made from 15 different herbs and spices was used.. The feature of the nutraceutical process is a novel water-based extraction of active ingredients to form aqueous moieties that address these complex processes. These extracts are known to provide anti-inflammatory, antioxidant, Dermal Papilla (DP) cells stimulation, follicle strengthening amino acids, and dihydrotestosterone (DHT)-inhibiting properties. In this clinical study the test product was evaluated using Trichoscan® and Dermatological assessments to assess the efficacy of the Diabliss HWS in providing hair fall control along with hair growth benefits as adjuvant therapy.
Aims and Objectives
To assess the impact of oral consumption of Diabliss Hair Water, a multi-component herbal water supplement (HWS) on hair fall reduction, hair growth, hair density, hair thickness
Materials and Methods
The study was designed as a pilot, exploratory, investigator driven single group clinical trial. Prior clearance from an independent ethics committee followed by written informed consent was taken from each study participants before any study related activities. A total of 40 male and female subjects (in 1:3 ratio) between the ages of 35-50 years, who satisfied the inclusion and exclusion criteria were enrolled in the study with 36 subjects completing the study. Based on the primary end-points and overall treatment duration a sample size of more than 33 subjects was sufficient to achieve statistical significance with 80% power and 5% level of significance. The sample size was calculated considering the Trichoscan® hair density data generated in previously conducted hair growth studies. The trial was registered on the clinical trial registry of India (CTRI) website at http://ctri.nic.in/ (CTRI number: CTRI/2020/ 03/023682).
The study was conducted for a period of 6 months for each subject and included a total of 9 visits. On the first visit, the subjects were screened on the study inclusion-exclusion criteria and blood samples were collected to check CBC and thyroid condition. Each assessment cycle encompassed two visits, 2 days apart as per the TrichoScan® SOP. On the first day of an assessment cycle, a small area of about 1.5cm in diameter was shaved and tattooed in the center. The assessments were performed in the same area at +2 days with TrichoScan®. The final eligibility was confirmed with density evaluation two days after shaving, i.e., on day 3. The selected subjects were provided with the Diabliss HWS along with a neutral shampoo for use. Along with daily consumption of the HWS, subjects were also advised to continue their normal nutrition and lifestyle.
Follow up evaluations were performed on day 60 & 62 (Week 8), day 120 &122 (Week 16), day 178 & 180 (Week 24). On the first visit of every assessment cycle, dermatological assessment, digital imaging was performed along with subject self-assessment questionnaire.
Subjects were asked to report any discomfort or adverse event, after starting the treatment on every follow-up visit. Subjects were assessed for overall change in health and fitness levels. Subjects were assessed for other health parameters including appetite, bowel movements, digestion, fatigue, stress level etc.
Assessment Details
Clinical evaluations were conducted with various established instruments along with clinical dermatological assessments for hair improvement:
Comb test
Hair pull test by a dermatologist
Hair thinning measurement on a photo numerical scale by a dermatologist
TrichoScan® assessments including Hair Growth Rate, Hair density, A:T ratio etc under phototrichogram assessment using Cutiscope on TrichoScan® software
Hair tensile strength using Chatillon Motorized Force Tester
Hair thickness by Denolite camera using Caslite Software
Test product
The HWS was prepared by Diabliss Consumer Products from a combination of water-based extracts of the following ingredients with known hair care benefits:
Sweet Potato (Ipomoea batatas),[8], [9], [10] Snake Gourd (Trichosanthes Anguina L),[4], [11], [12], [13] False Daisy (Eclipta prostrata), [14], [15], [16] Curry Leaves (Murraya koenigii),[17], [18], [19] Indian Gooseberry (Phyllanthus emblica),[20], [21], [22] Almond (Prunus dulcis),[23], [24] Walnut (Juglans regia),[24] Groundnut (Arachis hypogaea), [25] Flaxseed (Linum usitatissimum),[26], [27], [28] Chia Seed (Salvia hispanica), [29], [30] Lemon (Citrus x limon), [31], [32], [33] Chickpea (Cicer arietinum),[34] Cinnamon (Cinnamomum Zeylanicum Blume),[35], [36] Guava (Psidium guajava),[37], [38], [39] Cumin (Cumin cyminum),[40], [41] Spinach (Spinacia oleracea).[42]
Daily intake
5ml of Diabliss HWS was mixed in 500 ml of drinking water. 150 ml each was consumed at breakfast, lunch and dinner and balance 50 ml at bed time. Further, all other hair care regimens remained unchanged as per individual’s regular habit.
Statistical methodology
Statistical software R version 3.1.2 was used to analyse the data. Shapiro-Wilk Test was performed to check the normality of data. Paired t-test/Wilcoxon signed rank test for paired samples was performed for mean value comparison. P value <0.05 was considered to be significant
Results
|
Parameter |
Baseline |
Week 8 |
Week 16 |
Week 24 |
|
|
Hair Density (number of hair/cm2) |
Mean |
178.98 |
188.7 |
201.34 |
213.9 |
|
SD |
25.55 |
26.63 |
26.18 |
26.26 |
|
|
CFB |
- |
9.72** |
22.36** |
34.92** |
|
|
Hair growth rate (μm/ day) |
Mean |
273.7 |
287.36 |
311.53 |
331.76 |
|
SD |
36.44 |
37.19 |
30.43 |
30.49 |
|
|
CFB |
- |
13.66** |
37.82** |
58.06** |
|
|
Hair Thickness (μm) |
Mean |
40.54 |
46.31 |
49.59 |
53.02 |
|
SD |
4.61 |
4.54 |
3.96 |
4.19 |
|
|
CFB |
- |
5.77** |
9.05** |
12.48** |
|
|
Hair Tensile Strength (N) |
Mean |
0.69 |
0.72 |
0.76 |
0.78 |
|
SD |
0.04 |
0.03 |
0.03 |
0.03 |
|
|
CFB |
- |
0.03 |
0.07 |
0.1 |
|
|
Comb Test With Bulb (count) |
Mean |
31.54 |
20.35 |
10.86 |
7.43 |
|
SD |
13.47 |
10.58 |
5.13 |
3.85 |
|
|
CFB |
- |
11.19** |
20.68** |
24.11** |
|
|
Comb Test Without Bulb (count) |
Mean |
16.46 |
12.73 |
7.62 |
5.24 |
|
SD |
8.27 |
6.65 |
4.45 |
3.08 |
|
|
CFB |
- |
3.73** |
8.84** |
11.22** |
|
|
Hair Pull Test (score, where lower value is a sign of improvement) |
Mean |
2.57 |
2.24 |
1.66 |
0.84 |
|
SD |
0.43 |
0.37 |
0.39 |
0.43 |
|
|
CFB |
|
0.32** |
0.91** |
1.73** |
|
|
Hair Texture (score, where higher value is a sign of improvement) |
Mean |
2.43 |
3.00 |
4.19 |
4.70 |
|
SD |
0.50 |
0.00 |
0.40 |
0.46 |
|
|
CFB |
- |
0.57 |
1.76 |
2.27 |
|
|
Hair Shine (score, where higher value is a sign of improvement) |
Mean |
2.08 |
3.00 |
4.14 |
4.51 |
|
SD |
0.28 |
0.00 |
0.35 |
0.51 |
|
|
CFB |
- |
0.92 |
2.05 |
2.43 |
|
|
Hair Thinning, (On MSCR photonumerical scale, modified linear scale based on Norwood/Ludwig scale) |
Mean |
3.62 |
3.42 |
3.07 |
2.81 |
|
SD |
1.01 |
1.02 |
1.04 |
0.97 |
|
|
CFB |
- |
0.2 |
0.55 |
0.81 |
[Table 1] summarizes average values of various parameters investigated during the clinical trial. The data clearly demonstrates significant improvement in hair density, hair growth rate, hair thickness, hair tensile strength, hair loss reduction as measured by number of hairs from Comb test with bulb and comb test without bulb, hair pull, hair shine, hair texture and hair thinning characteristics.

As per Dermatological assessment ([Figure 1]), a statistically significant improvement in hair texture and hair shine in comparison to baseline was observed as early as 8 weeks and was noted to be progressive till the end of the study. A significant improvement in hair thinning (using a 10-point photo numerical linear in-house scale) in comparison to baseline was also observed as early as 8 weeks and progressive till the end of the study.
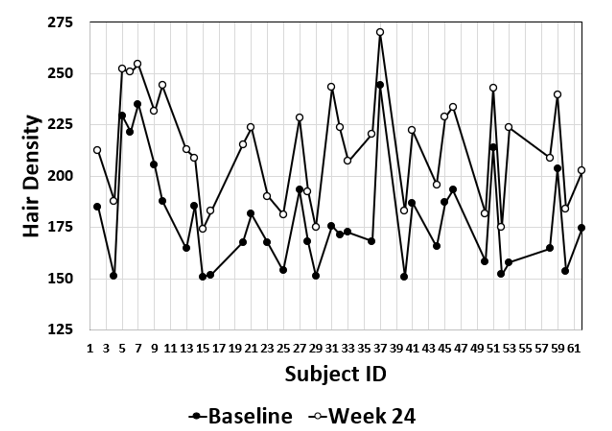
Significant improvement in hair growth rate, hair density in comparison to baseline was observed as early as 8 weeks and was noted to be progressive till the end of the study.
Significant improvement in hair thickness was noted in comparison to the baseline as early as 8 weeks and was noted to be progressive till the end of the study.
Significant improvement in hair strength by tensile test in comparison to the baseline, was observed as early as 8 weeks and was noted to be progressive till the end of the study.
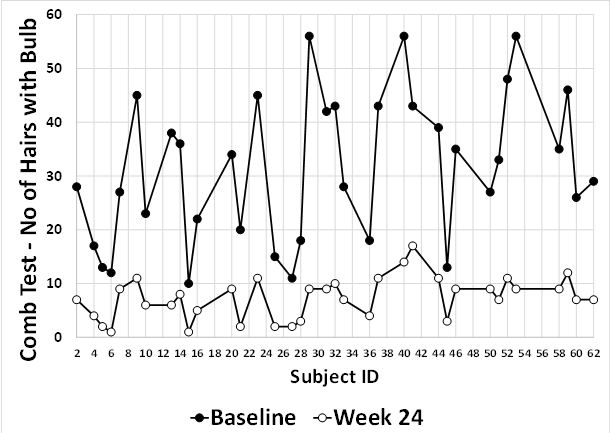
Significant Hair fall reduction by hair pull and hair comb test in comparison to the baseline was observed as early as 8 weeks and was noted to be progressive till the end of the study.
Review of the individual values for the clinical trial participants for these parameters are summarised in [Figure 2], [Figure 3], [Figure 4], [Figure 5]. Hair Density, Hair Growth Rate, Hair Tensile Strength and Hair Fall with Bulb are plotted in [Figure 2], [Figure 3], [Figure 4], [Figure 5] respectively and confirm that all subjects showed a positive response to the Diabliss HWS intervention.


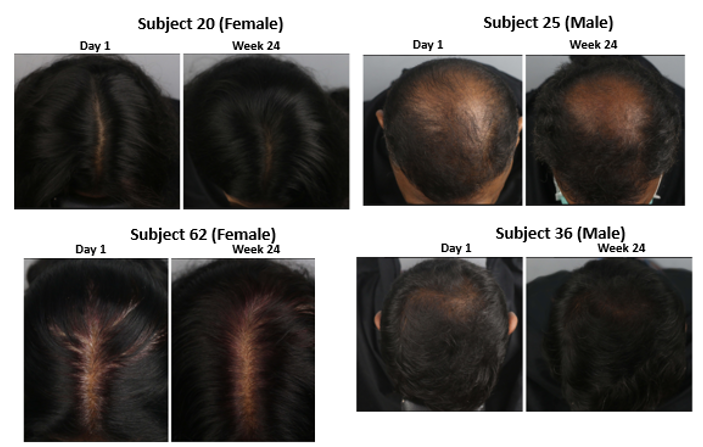
Male & Female Subjects Hair Coverage
Male and female subject’s hair coverage was photographed during each visit. Fig 6 and 7 shows representative female and male subjects hair coverage at the start and end of the trail showing visible improvement in the hair coverage.

Feedback Questionnaire Summary
|
N=37 |
Options |
Week 8 |
Week 16 |
Week 24 |
|
Do you feel that your hair length has improved since you have started using the product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Do you feel that your hair density has improved since you have started using the test product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Do you feel that you have more voluminous hair since you have started using the product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Do you feel that there is an improvement in the hair thickness since you have started using the product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Do you feel that your hair fall has reduced since you have started using the test product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Do you feel that your hair is healthier since the time you have used the product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Do you feel that your hair is softer since the time you have used the product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Do you feel that your hair shine has improved since the time you have used the product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Do you feel that you have bouncy hair since the time you have used the product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
|
|
Are you satisfied with the product? |
Agree |
100% ** |
100% ** |
100% ** |
|
Disagree |
0% |
0% |
0% |
As summarized in [Table 2], 100% of the study population agreed that with continuous intake of the Diabliss HWS, there was an improvement in Hair length, Hair Density, Hair Volume, Hair thickness and a reduction in Hair Fall. The test product had no side effects on the general well-being of the study population.
There was no product related AE/SAE or local intolerance of clinical significance.
Conclusions
There are various literature documenting the efficacy of various ingredients in the present formulation from Ayurveda to current medicine, as an individual ingredient as well as when used in combination. Nutraceutical products and supplements containing herbal ingredients are present in various forms. Herbal ingredients are also present in topical forms, however this product is prepared with a novel technique to provide the supplements in higher concentrations with an ease of its consumption. The study was planned to explore the benefits of the Diabliss HWS as a nutraceutical herbal adjuvant supplement for hair fall, hair growth and overall hair quality.
The product was found to be efficacious in providing hair fall control and hair growth benefits. The test product has improved organoleptics, making it easy to consume with no intolerance and AE reported in the study.
Regular consumption of HW water was found to be efficacious in various parameters as summarised below:
Increase in hair growth rate and density as per TrichoScan® assessment.
Increase in hair thickness as per Caslite software.
Increased hair tensile strength.
Reduction in hair fall.
Improvement in hair texture and hair shine.
Improvement was noted in the entire population in all the measured assessments, i.e., hair growth, hair density, hair thickness, hair tensile strength and hair fall reductions. Dermatologist assessment parameters of hair texture and hair shine also showed improvement in 100% of the subjects when compared to the baseline. There was no product related AE/SAE or local intolerance of clinical significance. This initial pilot exploratory study demonstrate that the Diabliss HWS shows a significant impact on hair fall reduction, hair quality and growth attributes. Further, placebo and regimen controlled clinical studies are recommended to evaluate long term safety and efficacy of the HWS.
Source of Funding
No external funding was received to carry out this work.
Conflict of Interest
None.
References
- David A Whiting. Chronic telogen effluvium. Dermatologic clinics 1996. [Google Scholar] [Crossref]
- EA Olsen, U Blume-Peytavi, A Tosti, R Trüeb. Female pattern hair loss. Female Pattern Hair Loss 2008. [Google Scholar] [Crossref]
- A Tosti, BM Piraccini. Diagnosis and Treatment of Hair Disorders An Evidence-Based Atlas. 1st Edn. 2005. [Google Scholar]
- Pereira, J Marcos.. Propedêutica das doenças dos cabelos e do couro cabeludo. São Paulo; Atheneu 2001. [Google Scholar]
- A Fahham, N Shamim, U Farooque, H Sheikh, R Aqeel. Telogen Effluvium: A Review of the Literature. Cureus 2020. [Google Scholar] [Crossref]
- AG Messenger, J Rundegren. rundegren J. Minoxidil: mechanism of action on hair growth. Br J Dermatol 2004. [Google Scholar] [Crossref]
- KS Houschyar, MR Borrelli, C Tapking, D Popp, B Puladi, M Oom. Molecular mechanisms of hair growth and regeneration: current understanding and novel paradigms. Dermatology 2020. [Google Scholar] [Crossref]
- HM Almohanna, AA Ahmed, JP Tsatalis, A Tosti. The role of vitamins and minerals in hair loss: a review. Dermatol Ther (Heidelb) 2019. [Google Scholar]
- A.C Ross, B Caballero, R.J Cousins, K.L Tucker. Modern nutrition in health and disease. 11th Edn. 2012. [Google Scholar]
- J Combs, F Gerald, JP Mcclung. The vitamins: fundamental aspects in nutrition and health. 2016. [Google Scholar]
- ND Devi. Medicinal values of Trichosanthus cucumerina L.(snake gourd)-a review. J Pharm Res Int 2017. [Google Scholar]
- S Sandhya. 12.Sandhya, S., et al. "Potentiality of aqueous leaf extract of Trichosanthes cucumerina Linn. on hair growth promotion in Wistar albino rats. India J Nat Prod Resour 2012. [Google Scholar]
- V P Rekha. Review on trichosanthes species: Deserving potential pharmacological properties and boon in medical applications. Am J Ethnomed 2015. [Google Scholar]
- Shahnaz Begum. Comparative hair restorer efficacy of medicinal herb on nude (Foxn) mice. BioMed Res Int 2014. [Google Scholar] [Crossref]
- J Haiyan. Treatment of 21 cases of alopecia with Shengfa Wan. Sichuan J Tradit Chinese Med 1987. [Google Scholar]
- A Madan, A Arun, S Verma. A non-comparative open label pilot study to see the efficacy and consumer response of vegetal hair well in preventing hair fall and promoting hair growth. Int J 2014. [Google Scholar]
- J Shinde. Advances in disease protecting ingredient of Murraya Koenigii (Curry Leaves)-A textual Herbal Medicine with newer Approach. Int J Innov Pharm Sci Res 2016. [Google Scholar]
- R Balakrishnan, D Vijayraja, J Song-Hee, P Ganesan, I Su-Kim, C Dong-Kug. Medicinal profile, phytochemistry, and pharmacological activities of Murraya koenigii and its primary bioactive compounds. Antioxidants (Basel) 2020. [Google Scholar] [Crossref]
- Satish Saini, Gopu Bala Show Chand, Reddy. A review on curry leaves (Murraya koenigii): Versatile multi-potential medicinal plant. Am J Phytomed Clin Ther 2015. [Google Scholar]
- H Dur-Zong, C Yi-Wei, L Ming-Yie, C Pei-Yi, S Periasamy. Protective effect of 3, 4-methylenedioxyphenol (sesamol) on stress-related mucosal disease in rats. BioMed Res Int 2013. [Google Scholar] [Crossref]
- M Majeed, K Nagabhushanam, L Mundkur, P Neupane, K Shah. Clinical study to evaluate the efficacy and safety of a hair serum product in healthy adult male and female volunteers with hair fall. Clin, Cosmet Investig Dermatol 2020. [Google Scholar] [Crossref]
- S Luanpitpong. Emblica (Phyllanthus emblica Linn.) fruit extract promotes proliferation in dermal papilla cells of human hair follicle. Res J Med Plant 2011. [Google Scholar]
- H Hajimehdipoor, N Nikmanesh, M Mohammadi-Motamed. Amla oil, a pharmaceutical product based on traditional knowledge for alopecia treatment. Res J Pharmacogn 2019. [Google Scholar] [Crossref]
- R Suraja, G Rejitha, JAJ Sunilson, K Anandarajagopal, P Promwichit. In vivo hair growth activity of Prunus dulcis seeds in rats. Biol Med 2009. [Google Scholar]
- N Thebo, A Simair, Wazir Sheikh, AR Abbasi, L A Jabbar, HM Nizamani. Clinical study of the prunus dulcis (Almond) shell extract on tinea capitis infection. Nat Prod Chem Res 2014. [Google Scholar]
- Ali Jahanban-Esfahlan. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int J Mol Sci 2019. [Google Scholar] [Crossref]
- A Therapeutics. A Study in Male and Female Subjects With Androgenetic Alopecia Treated With ATI-50002 Topical Solution. . [Google Scholar]
- K Beroual, Z Maameri, S Halmi, B Benleksira, A Agabour, YH Pacha. Effects of Linum usitatissimum L. ingestion and oil topical application on hair growth in rabbit. Int J Med Arom Plants 2013. [Google Scholar]
- W O'Neill, S Mckee, AF Clarke. Flaxseed (Linum usitatissimum) supplementation associated with reduced skin test lesional area in horses with Culicoides hypersensitivity. Can J Vet Res 2002. [Google Scholar]
- A Goyal, V Sharma, N Upadhyay, S Gill, M Sihag. Flax and flaxseed oil: an ancient medicine & modern functional food. J Food Sci Technol 2014. [Google Scholar] [Crossref]
- NM Ali, SK Yeap, WY Ho, BK Beh, SW Tan, SG Tan. The promising future of chia, Salvia hispanica L. J Biomed Biotechnol 2012. [Google Scholar] [Crossref]
- R Ullah, M Nadeem, A Khalique, M Imran, S Mehmood, A Javid. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): a review. J Food Sci Technol 2016. [Google Scholar] [Crossref]
- M Klimek-Szczykutowicz, A Szopa, H Ekiert. Citrus limon (Lemon) phenomenon-a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020. [Google Scholar] [Crossref]
- Noura S Dosoky, William N Setzer. Biological activities and safety of Citrus spp. essential oils. Int J Mol Sci 2018. [Google Scholar] [Crossref]
- Q Tamara, Z Umber, R Rafia, M Muhammad, S Sadia, N Shafaq. Lemon as a source of functional and medicinal ingredient: A review. Int J Chem Biochem Sci 2018. [Google Scholar]
- TC Wallace, R Murray, KM Zelman. The nutritional value and health benefits of chickpeas and hummus. Nutrients 2016. [Google Scholar] [Crossref]
- W Tung-Chou, L Yuan-Sheng, K Rajamani, H Horng-Jyh, L Shinn-Zong, C Tzyy-Wen. Effect of Cinnamomum osmophloeum Kanehira leaf aqueous extract on dermal papilla cell proliferation and hair growth. Cell Transplant 2018. [Google Scholar] [Crossref]
- L Indriana, W Pangkahila, IGMade Aman. Topical application of cinnamon (cinnamomum burmanii) essential oil has the same effectiveness as minoxidil in increasing hair length and diameter size of hair follicles in male white Wistar rats (rattus norvegicus). IJAAM (Indonesian J Anti-Aging Med) 2018. [Google Scholar]
- Díaz-De-Cerio, Elixabet. Health effects of Psidium guajava L. Leaves: An overview of the last decade. In J Mol Sci 2017. [Google Scholar] [Crossref]
- PG Daswani, S Manasi, MS Gholkar, TJ Birdi. Psidium guajava: A single plant for multiple health problems of rural Indian population. Pharmacogn Rev 2017. [Google Scholar] [Crossref]
- NP Gavatia. Therapeutic potential of Psidium guajava and its polyherbal formulation on chemotherapy induced alopecia. J Pharm Res 2011. [Google Scholar]
- K Srinivasan. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: traditional uses, chemical constituents, and nutraceutical effects. Food quality and safety 2018. [Google Scholar]
How to Cite This Article
Vancouver
Janaki C, Sachdev M, Ritambhara . A single arm, exploratory and pilot study to evaluate the efficacy of a multi-component water-based herbal supplement in hair growth, density, follicle strength and hair fall in healthy male and female subjects [Internet]. IP Indian J Clin Exp Dermatol. 2025 [cited 2025 Sep 08];7(3):249-255. Available from: https://doi.org/10.18231/j.ijced.2021.047
APA
Janaki, C., Sachdev, M., Ritambhara, (2025). A single arm, exploratory and pilot study to evaluate the efficacy of a multi-component water-based herbal supplement in hair growth, density, follicle strength and hair fall in healthy male and female subjects. IP Indian J Clin Exp Dermatol, 7(3), 249-255. https://doi.org/10.18231/j.ijced.2021.047
MLA
Janaki, C.S, Sachdev, Mukta, Ritambhara, . "A single arm, exploratory and pilot study to evaluate the efficacy of a multi-component water-based herbal supplement in hair growth, density, follicle strength and hair fall in healthy male and female subjects." IP Indian J Clin Exp Dermatol, vol. 7, no. 3, 2025, pp. 249-255. https://doi.org/10.18231/j.ijced.2021.047
Chicago
Janaki, C., Sachdev, M., Ritambhara, . "A single arm, exploratory and pilot study to evaluate the efficacy of a multi-component water-based herbal supplement in hair growth, density, follicle strength and hair fall in healthy male and female subjects." IP Indian J Clin Exp Dermatol 7, no. 3 (2025): 249-255. https://doi.org/10.18231/j.ijced.2021.047
