- Visibility 82 Views
- Downloads 5 Downloads
- DOI 10.18231/j.ijced.2025.024
-
CrossMark
- Citation
Study of efficacy of Rapamycin 0.1% cream in facial Angiofibroma – Case series
- Author Details:
-
Anirudha Gulanikar
-
Asim Ilyas Patel *
-
Aishwarya Gulanikar
Introduction
Tuberous sclerosis complex (TSC) is an multisystemic neurocutaneous disease with autosomal dominant inheritance, characterized by hamartomas affecting skin, heart, kidney, lung, eye, and central nervous system. Despite being autosomal dominant, 70% cases are sporadic.[1] TSC affects one in every 6,000 to 10,000 newborn babies with no gender and race predilection. Its recognition is difficult sometimes because of phenotypic variability. [2], [3]
Pathogenesis underlying TSC is mutation in TSC1 and TSC 2 genes which are tumour suppressor genes coding for hamartin and tuberin proteins respectively. These proteins normally suppress mTOR (mammalian target for rapamycin) pathway resulting in a permanent activation of the pathway. The overactive mTOR pathway in turn results in unregulated cellular proliferation and protein synthesis resulting in multiorgan hamartoma formation. [4], [5]
The cutaneous manifestations of TSC include facial angiofibroma, cephalic plaques, hypomelanotic macules or confetti-like lesions, periungual and intraoral fibromas and shagreen patch. Facial angiofibroma(adenoma sebaceum) are small, symmetrical, pink-red to brown papules which coalesce to form plaques. They are composed of blood vessels and fibrous tissue. [6]
Angiofibroma is considered as one of the major diagnostic criteria according to the recommendations given by “International Tuberous Sclerosis Complex Consensus Conference 2012”. [7] They are seen in 83 to 90% of cases and usually appear in the first decade, mostly around 3rd and 4th year of life. The number of lesions can increase during adolescence and stabilise in adulthood. [2] They are usually asymptomatic but can bleed spontaneously. They can impair vision and affect the quality of life due to the facial disfigurement.[8] Radiofrequency ablation, cryotherapy, dermabrasion, laser treatment, podophyllotoxin application and surgical removal of large lesions are some of the therapeutic options available to patients. [9]
Rapamycin (Sirolimus) is a molecule that belongs to a group known as mTOR inhibitors. Antiproliferative effect of this drug is attributed to its ability to normalize increased mTOR signaling in tumor cells. It also decreases the levels of Vascular Endothelial Growth Factor (VEGF), thus inhibit angiogenesis. [10] Rapamycin is the drug that inhibit mTOR pathway and may be useful for treatment of various clinical manifestation of TSC. Various investigators have used different concentrations of topical rapamycin in the treatment of facial angiofibroma. Our study is an addition to those studies. [11], [12]
Aim
The aim of our study was to assess effectiveness and safety of rapamycin 0.1% cream for facial angiofibroma in patients with tuberous sclerosis in Indian population.
Materials and Methods
This study is case series involving 5 patients with facial angiofibroma fulfilling the criteria for tuberous sclerosis complex. There was loss to follow up of 1 patient thus, is excluded from the study analysis. The project was carried out in tertiary care centre in Maharashtra. Due approval was taken from institutional ethics committee before commencing the study. Before enrolling participants, a written informed consent was taken in patients’ vernacular language. Baseline investigation included complete blood count, urine routine, chest X-ray, ultrasonography of abdomen and pelvis and MRI brain. Pre-treatment clinical pictures were obtained. Patients were asked to apply rapamycin 0.1% cream every night and asked to follow up each month for 6 months. At 3 and 6 months’ follow up patients were asked a Global question (GQ) and assessed using FASI tool. GQ is self-assessment of skin disease severity on 0-10 visual analogue scale, where 0 indicates clear skin and 10 indicates worst possible condition. Facial Angiofibroma Severity Index (FASI) (Table 1) is a reliable tool to measure severity and responsiveness to treatment in facial angiofibroma. [13] Interpretation of FASI score is that ≤ 5 is considered mild, 6-7 is moderate, ≥ 8 is considered a severe disease. Analysis of efficacy of therapy was done by comparing pretreatment, 3 month and 6-month post treatment FASI and GQ scores.
Patient A
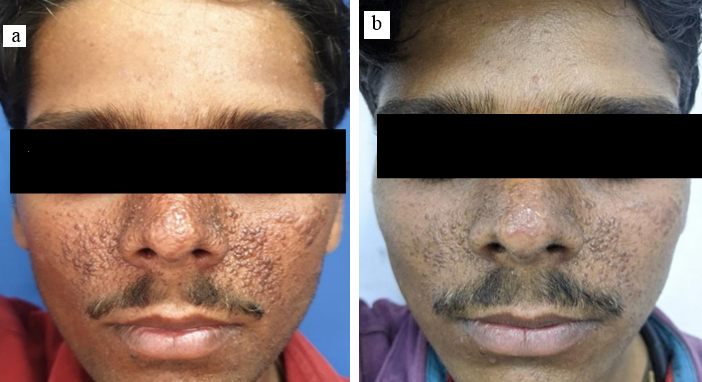
24 years old male patient presented with facial angiofibroma since 1 and a half year with no significant family history. The FASI score for severity was 8 and GQ score 10 on initial examination indicating severe adenoma sebaceum. ([Figure 1]a) The patient also showed hypomelanotic papules, shagreen patch, fibrous cephalic plaque and confetti skin lesions all of which are part of diagnostic criteria for TSC. Biochemical analysis of the serum in form of RFT, LFT, serum electrolytes and urine routine and microscopy were found normal. Radiological examination revealed loss of corticomedullary differentiation in kidneys, subependymal nodules in bilateral lateral ventricles and cortical tubers in fronto-parietotemporal region. These systemic findings are known to be associated with TSC. Thus, the diagnosis of TSC was confirmed as the diagnostic criteria was fulfilled. Patient was then prescribed rapamycin 0.1% cream topical application and followed up till 6 months. Response was noticed after 2 months of application. At 3 months of use the FASI was 7 and GQ was 7 showing an 11% and 30% reducing respectively. At the 6 months follow up he showed a FASI of 6 (reduction of 22%) and GQ of 4 (reduction of 60 %). ([Figure 1]b) During the course the patient did not complain of any side effect or any derangements in blood and urine examination.
Patient B
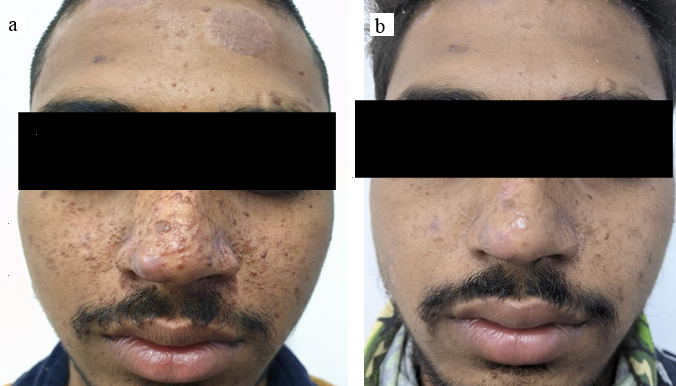
18 years old male patient presented with facial angiofibroma present for over 5 years with history of previous unsuccessful surgical treatment. The FASI score for severity was 7 and GQ score 9 on initial examination indicating moderate severity of adenoma sebaceum. ([Figure 2]a) Cutaneous examination showed presence of fibrous cephalic patch and shagreen patch. MRI of the brain showed cortical tubers and subependymal nodules. USG showed hemangioma of left lobe of liver and angiomyolipoma in kidney. Therefore, the patient fulfilled the criteria for TSC. Biochemical analysis of the serum in form of RFT, LFT, serum electrolytes and urine routine and microscopy were found normal. Topical treatment with rapamycin started with once daily application. The patient had stopped treatment for 1 month resulting slight increase in size of lesions however at 3 months after restartingtreatment the FASI was 6 (decreasing by 11%) and GQ was 6 (decreasing by 30%). At the 6 months follow up he showed a FASI of 4 (reduction of 33%) and GQ of 3 (reduction of 60 %). ([Figure 2]b) During the course neither did the patient complain of any side effect nor were any derangements noted in blood and urine examination.
Patient C
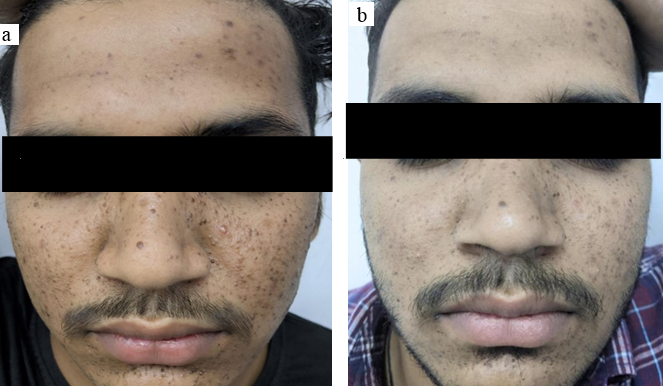
17 years old male came to the OPD with history of facial angiofibroma since 5 years. The FASI score for severity was 6 and GQ score 8 on initial examination portraying moderate severity adenoma sebaceum. ([Figure 3]a) Head to toe examination showed presence of shagreen patch and intraoral fibromas. Radiological examination showed cortical tubers and subependymal nodules. Thus, the patient fulfils criteria for TSC. Biochemical analysis of the serum in form of RFT, LFT, serum electrolytes and urine routine and microscopy were found normal. Response first appeared 3 months later with the FASI was 5 (11% fall) and GQ was 5 (20% fall). At the 6 months follow up he showed a FASI of 4 (22% fall) and GQ of 3 (50% fall). ([Figure 3]b) The patient complained of skin irritation after application of cream which resolved after application of hydrocortisone cream for 1 week. Local and systemic examination during course of treatment showed no complications to treatment.
Patient D
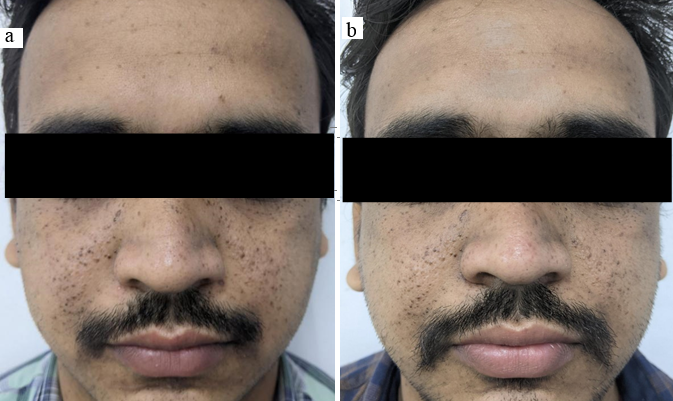
27 years old male patient presented with facial angiofibroma since 15 years. The FASI score for severity was 6 and GQ score 8 on initial examination meaning he had moderately severe adenoma sebaceum. ([Figure 4]a) General examination showed ungal fibroma, shagreen patch and intraoral fibroma. Radiological examination showed subependymal nodules. Therefore, the patient fulfilled the diagnostic criteria for TSC. Biochemical analysis of the serum in form of RFT, LFT, serum electrolytes and urine routine and microscopy were found normal. 3 months into treatment response was first noted with the FASI was 5 (reduction of 11%) and GQ was 5 (reduction of 20%). At the 6 months follow up he showed a FASI of 5 (reduction of 11%) and GQ of 3 (reduction of 50 %). ([Figure 4]b) Patient showed local irritation and burning sensation after rapamycin application. He was prescribed hydrocortisone cream for 1 week after which the symptoms resolved. No other local or systemic complications were noted.
Results
All 4 patients were male and had mean age of 22 years. All patients showed both systemic and cutaneous manifestations of TSC. Shagreen patch was most common among the cutaneous and subependymal nodules were most common among the systemic manifestations seen. Two patients had local irritation and burning sensation after application of rapamycin cream initially. They were prescribed hydrocortisone 0.1% cream for 1-2 weeks. All patients tolerated treatment very well. Mean response time to treatment was found to be 2.5 months.
|
Components |
Grade |
|||
|
0 |
1 |
2 |
3 |
|
|
Erythema |
- |
Light red |
Red |
Dark red |
|
Size |
- |
Small < 5mm |
Large ≥ 5mm |
Confluent |
|
Extension |
- |
- |
< 50% cheek surface area |
≥ 50% cheek surface area |
|
FASI score |
Mean difference |
Mean percentage change (%) |
|
Base vs 3 months follow up |
1.0 |
↓14.81 |
|
Base vs 6 months follow up |
2.0 |
↓29.62 |
|
GQ score |
Mean difference |
Mean percentage change (%) |
|
Base vs 3 months follow up |
3.0 |
↓34.28 |
|
Base vs 6 months follow up |
5.5 |
↓62.86 |
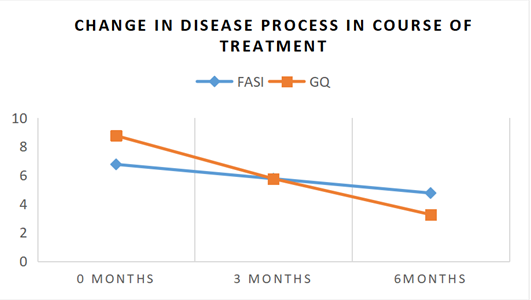
All patients showed improvement in the facial angiofibroma after treatment. mean FASI score before therapy was 6.75 and mean GQ score was 8.75. Post-treatment, they reduced to 4.75 and 3.25 respectively. At 3 months the mean decrease in FASI was 14.81% and for GQ was 34.28%. Follow up after 6 months showed mean decrease of FASI by 29.62% (p=0.0255) and GQ by 62.86% (p=0.0001). (Tables 2,3) ([Figure 5]). At the end of the study all the participants had FASI score ≤ 4 indicating a mild disease process.
Discussion
Tuberous sclerosis complex is an autosomal dominant disorder which is characterized by benign hamartomatous growth in multiple tissues of the body. Facial angiofibroma is a frequent manifestation of this disorder.
Salido R et al included 10 men in their study with a mean age of 13 with a range of 6- 43 where as our study included 4 men with a mean age of 22 with a range of 18-27 show casing the impact of treatment in a younger spectrum of patients.[6] In a study by Koenig MK et al the participants included 46% females and 54% showing a more uniform population and better generalizability as compared to the other studies including ours which had maximum male participants.[14]
We used a specially compounded formulation of 0.1 % rapamycin for topical application. In the previous studies like EXIST-1,2 trails oral mTOR inhibitors were used and in others 1 %, 0.4%, 0.003%,0.015% formulations were used. [6], [11], [14], [15] Haemel AK et al. used twice a day application, whereas Salido et al. use once daily 3 days in a week application both showing positive results. Our study used once daily application showing a smaller quantity applied daily produced similar results along with avoiding dose dependent adverse effects of the drug.
In the study by Koenig MK et al 73% in the treatment group and 38% in the placebo group showed response to treatment measured subjectively.Salido R et al found a decrease in FASI by 60.2% after a span of 6 months with treatment. Our study combined both these approaches to find efficacy of this treatment showing a mean decrease of FASI by 29.62% and decrease in GQ by 55%. Mean response time for all participants in our study was 2.5 months which corresponds to the finds of all other similar studies.
Participants in our study did not report any systemic adverse effect in the duration of use. Local irritation was noted in 2 participants which resolved after treatment. One study reported an episode of GTCS following topical use that resulted in aspiration pneumonia but this was an isolated event.[14]
Jin Park et al found that for lesions >4 mm needs additional laser therapy for optimum response along with topical rapamycin. We used only topical therapy as mode of management in our study.[15]
Topical rapamycin is thus found to be an effective intervention with limited adverse effects in patients with facial angiofibroma. Stopping of therapy is associated relapse. It remains to be seen whether long-term therapy leads to continued improvement
Conclusion
Facial angiofibroma is major cosmetic concern for patients of TSC.Topical rapamycin appears to be safe and efficacious option to consider for treatment. It is a good non-invasive modality, although continued therapy for longer duration may be needed. Combination of topical therapy with other modalities like laser treatment may be considered. Further multicentric long term studies are required for assessing full extent of the use of topical mTOR inhibitors in facial angiofibroma.
Limitations
Stability and efficacy of compounded preparation of rapamycin cannot be ensured.
High cost of medication. Compounding pharmacies are not easily accessible.
Systemic drug levels could not be measured. More studies are required to evaluate systemic absorption of topically applied rapamycin and to determine safety of the same.
Small number of patients.
Multicentric trials would have been better option as getting large number at on centre is difficult.
Conflicts of Interest
None.
Source of Funding
None.
References
- DA Rodrigues, CM Gomes, IMC Costa. Tuberous sclerosis complex. An Bras Dermatol 2012. [Google Scholar]
- K Sadowski, K Kotulska, RA Schwartz, S Jóźwiak. Systemic effects of treatment with mTOR inhibitors in tuberous sclerosis complex: A comprehensive review. J Eur Acad Dermatol Venereol 2016. [Google Scholar]
- FJ Dimario, M Sahin, D Ebrahimi-Fakhari. Tuberous Sclerosis Complex. Pediatr Clin North Am 2015. [Google Scholar]
- LKL Portocarrero, KN Quental, LP Samorano, ZNP Oliveira, MCDM Rivitti-Machado. Tuberous sclerosis complex: review based on new diagnostic criteria. An Bras Dermatol 2018. [Google Scholar]
- JW Wheless, H Almoazen. A novel topical rapamycin cream for the treatment of facial angiofibromas in tuberous sclerosis complex. J Child Neurol 2013. [Google Scholar]
- R Salido, G Garnacho-Saucedo, I Cuevas-Asencio, J Ruano, M Galán-Gutierrez, A Vélez. Sustained clinical effectiveness and favorable safety profile of topical sirolimus for tuberous sclerosis - Associated facial angiofibroma. J Eur Acad Dermatol Venereol 2012. [Google Scholar]
- H Northrup, DA Krueger, S Roberds, K Smith, J Sampson, B Korf. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013. [Google Scholar]
- R Balestri, I Neri, A Patrizi, L Angileri, L Ricci, M Magnano. Analysis of current data on the use of topical rapamycin in the treatment of facial angiofibromas in tuberous sclerosis complex. J Eur Acad Dermatol Venereol 2015. [Google Scholar]
- SL Cinar, D Kartal, AK Bayram, M Canpolat, M Borlu, A Ferahbas. Topical sirolimus for the treatment of angiofibromas in tuberous sclerosis. Indian J Dermatol Venereol Leprol 2017. [Google Scholar]
- SL Dabora, DN Franz, S Ashwal, A Sagalowsky, FJ DiMario Jr, D Miles. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF- D levels decrease. PLoS One 2011. [Google Scholar] [Crossref]
- AK Haemel, AL O’brian, JM Teng. Topical rapamycin: a novel approach to facial angiofibromas in tuberous sclerosis. Arch Dermatol 2010. [Google Scholar]
- RS Foster, LJ Bint, AR Halbert. Topical 0.1% rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: a pilot study of four patients. Australas J Dermatol 2012. [Google Scholar]
- R Salido-Vallejo, J Ruano, G Garnacho-Saucedo, E Godoy-Gijón, D Llorca, C Gómez-Fernández. Facial Angiofibroma Severity Index (FASI): reliability assessment of a new tool developed to measure severity and responsiveness to therapy in tuberous sclerosis-associated facial angiofibroma. Clin Exp Dermatol 2014. [Google Scholar]
- MK Koenig, AA Hebert, J Robertson, Ja Samuels, J Slopis, A Woerner. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double blind,randomised, controlled trail to evaluate safety and efficacy of topically applied rapamycin. Drugs R D 2012. [Google Scholar]
- J Park, SK Yun, YS Cho, KH Song, HU Kim. Treatment of angiofibromas in tuberous sclerosis complex: the effect of topical rapamycin and concomitant laser therapy. Dermatology 2014. [Google Scholar]
